Synovial Sarcoma Market
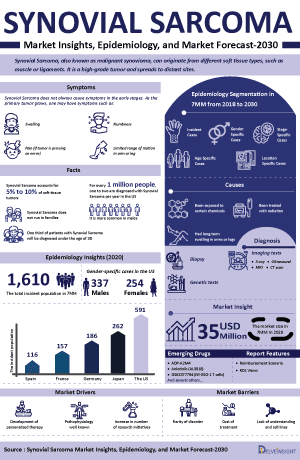
- The Synovial Sarcoma Incidence has been estimated to be very low and rare, accounting for only 5–10% of all soft tissue sarcoma. This means a small patient target for new entrants.
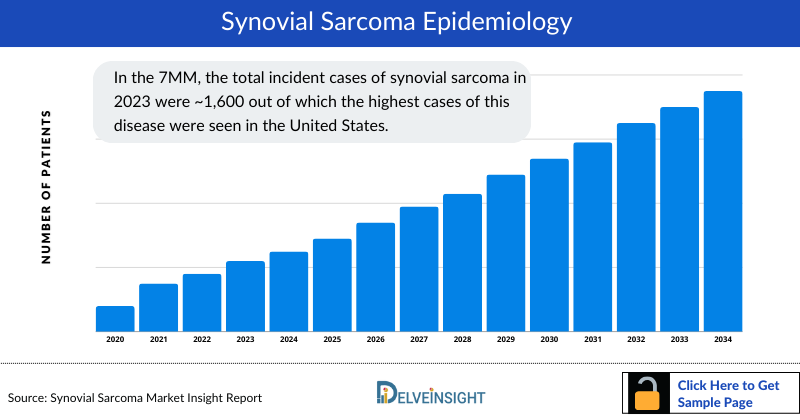
- In the 7MM, the total Synovial Sarcoma Incident Cases in 2023 were ~1,600 out of which the highest cases of this disease were seen in the United States.
- Synovial Sarcoma highly expresses cancer testis antigens, including NY-ESO-1 and MAGE-A4. NY-ESO-1 was one of the first antigens that was targeted with T-cell based technologies.
- Synovial Sarcoma primarily affects younger individuals, with one-third of patients diagnosed before the age of 30.
- TECELRA (afamitresgene autoleucel) is the first engineered cell therapy for a solid tumor cancer approved in the US, and the first new therapy option in more than a decade for Synovial Sarcoma. However, T-cell therapy is generally limited to specific sites within the United States that are able to administer this therapy.
- Synovial Sarcoma is a prime example of a cancer with limited immune cell presence, making traditional treatments like checkpoint inhibitors less effective. However, using a patient's genetically engineered immune cells to target the cancer shows promise. This approach highlights the early but exciting potential of T-cell therapy in advancing cancer treatment.
- Adaptimmune intends to begin a rolling Biologics License Application (BLA) submission for letetresgene autoleucel in 2025, targeting the treatment of Synovial Sarcoma. This move will strengthen Adaptimmune’s sarcoma portfolio by expanding the treatable patient population to include those with NY-ESO-1 positive MRCLS and Synovial Sarcoma solid tumors.
- The leading Synovial Sarcoma Companies such as Adaptimmune Therapeutics, Advenchen Laboratories, Takara Bio, OncoTherapy Science, and others, are currently working on developing therapies for Synovial Sarcoma.
Request for Unlocking the Sample Page of the Synovial Sarcoma Treatment Market
DelveInsight’s "Synovial Sarcoma Market Insights, Epidemiology, and Market Forecast – 2034" report delivers an in-depth understanding of Synovial Sarcoma, historical and forecasted epidemiology as well as the Synovial Sarcoma market trends in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
The Synovial Sarcoma Treatment Market Report provides current treatment practices, marketed and emerging drugs, Synovial Sarcoma market share of individual therapies, and current and forecasted Synovial Sarcoma market size from 2020 to 2034, segmented by seven major markets. The report also covers current Synovial Sarcoma treatment practices/algorithms and unmet medical needs to curate the best of the opportunities and assess the underlying potential of the market.
|
Study Period |
|
|
Forecast Period |
|
|
Geographies Covered |
|
|
Synovial Sarcoma Epidemiology |
Segmented by:
|
|
Synovial Sarcoma Companies |
|
|
Synovial Sarcoma Therapies |
|
|
Synovial Sarcoma Market |
|
|
Analysis |
|
Synovial Sarcoma Treatment Market: Understanding and Algorithm
Synovial Sarcoma, also known as malignant synovioma, is a rare form of cancer originating from various types of soft tissue, including muscles and ligaments. It frequently occurs in the arms, legs, or feet, particularly near joints such as the wrist or ankle. This cancer can also develop in the soft tissues of the lungs or abdomen. Synovial Sarcoma accounts for 5% to 10% of all soft-tissue tumors. It primarily affects young adults but can occur at any age. Symptoms often include swelling or a palpable mass, pain, and limited range of motion if near a joint.
Synovial Sarcoma Diagnosis
Synovial Sarcoma typically begins when the patient notices symptoms such as swelling, a noticeable mass, or pain in areas like the arm, leg, or near joints. They visit a primary care physician, who performs a physical exam and orders imaging tests, such as X-rays, MRI, or CT scans. Abnormal results from these scans lead to a referral to a specialist, such as an oncologist or orthopedic surgeon. The specialist conducts a biopsy to obtain a tissue sample from the tumor, which is then analyzed by pathologists to confirm the presence of Synovial Sarcoma. Once diagnosed, additional tests, like PET scans, are used to determine the cancer's stage. A multidisciplinary team then creates a tailored treatment plan, which may involve surgery, radiation therapy, and chemotherapy. The patient begins treatment and is closely monitored through regular follow-ups and imaging studies to gauge effectiveness and check for recurrence. Throughout the process, the patient receives support from healthcare providers, family, and support groups.
Further details related to diagnosis will be provided in the report…
Synovial Sarcoma Treatment
The primary treatment for Synovial Sarcoma involves surgery to remove the entire tumor along with surrounding healthy tissue, ensuring "clear margins" to maximize the likelihood of eradicating all cancer cells. Achieving clear margins can be challenging depending on the tumor's location and size, as it may impact the preservation of function. Radiotherapy may be used in conjunction with surgery, either before or after, to lower the risk of residual cancer cells. For advanced or metastatic Synovial Sarcoma, chemotherapy, typically with doxorubicin and/or Ifosfamide, may be recommended. However, due to its rarity, the role of chemotherapy in preventing metastases and improving survival is not fully agreed upon by experts. An oncologist will carefully weigh the potential benefits of chemotherapy against its possible side effects when creating a treatment plan.
Synovial Sarcoma Epidemiology
The Synovial Sarcoma epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by the total incident cases of Synovial Sarcoma, gender-specific cases of Synovial Sarcoma, age-specific cases of Synovial Sarcoma, location-specific cases of Synovial Sarcoma, stage-specific cases of Synovial Sarcoma, antigen-specific (MAGE-A4, NY-ESO-1, PRAME, and others) cases, and line-wise treated patient pool in the 7MM market covering the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan from 2020 to 2034.
- Among the 7MM, the US accounted for the highest Synovial Sarcoma Incident Cases in 2023, with around 650 cases; these cases are expected to increase during the forecast period.
- In 2023, the total number of Synovial Sarcoma Incident Cases in EU4 and the UK was highest in Germany, while the lowest number of cases was in Spain.
- In cases of gender-specific Synovial Sarcoma, males are observed to be the predominant contributors compared to females.
- As per DelveInsight’s estimates, the highest incidence of Synovial Sarcoma in Japan is in Stage II which has ~100 cases in 2023 that are projected to increase during the forecast period.
Unlock comprehensive insights! Click Here to Purchase the Full Epidemiology Report @ Synovial Sarcoma Prevalence
Synovial Sarcoma Drug Chapters
The drug chapter segment of the Synovial Sarcoma Therapeutics Market Report encloses a detailed analysis of the late-stage (Phase III), mid-stage (II), and early-stage (Phase I) pipeline drugs. The recent approval of TECELRA is a momentous step in Adaptimmune’s journey to redefine the way cancer is treated and the culmination of a decade of groundbreaking. The current emerging key players and their respective drug candidates include AL3818 (Advenchen Laboratories), Letetresgene autoleucel (Adaptimmune Therapeutics), and TBI-1301 (Takara Bio). The drug chapter also helps understand the Synovial Sarcoma clinical trial details, expressive pharmacological action, agreements and collaborations, approval, and patent details, and the latest news and press releases.
Synovial Sarcoma Marketed Drugs
- TECELRA (afamitresgene autoleucel): Adaptimmune Therapeutics
TECELRA (afami-cel; formerly ADP-A2M4) is a melanoma-associated antigen A4 (MAGE-A4)-directed genetically modified autologous T cell immunotherapy indicated for the treatment of adults with unresectable or metastatic Synovial Sarcoma who have received prior chemotherapy, are HLA-A*02:01P, -A*02:02P, -A*02:03P, or -A*02:06P positive and whose tumor expresses the MAGE-A4 antigen as determined by FDA-approved or cleared companion diagnostic devices. In August 2024, Adaptimmune Therapeutics received the US FDA accelerated approval of TECELRA for the treatment of adults with unresectable or metastatic Synovial Sarcoma. The approval of TECELRA was based on results of the SPEARHEAD-1 (Cohort 1) trial. TECELRA treatment resulted in an ORR of 43% with a complete response rate of 4.5%.
TECELRA is the first engineered cell therapy for solid tumors. Also, it is the first new treatment option for Synovial Sarcoma in more than a decade. Sarcoma centers of excellence across the Unites States are being onboarded as Authorized Treatment Centers (ATCs) for TECELRA.
Synovial Sarcoma Emerging Drugs
- Letetresgene autoleucel (lete-cel): Adaptimmune Therapeutics
Letetresgene autoleucel is an engineered TCR T-cell therapy targeting the solid tumor antigen NY-ESO-1. Lete-cel is being investigated for the treatment of Synovial Sarcoma and myxoid/round cell liposarcoma (MRCLS) in the pivotal IGNYTE-ESO (NCT03967223) trial in patients who received prior anthracycline treatment. Sponsorship of the ongoing clinical trials for lete-cel has transitioned from GSK to Adaptimmune. The IGNYTE-ESO trial, which is ongoing but no longer open for new participants, has had its interim analysis data presented at CTOS in 2023 also reported data for sub-study 1 of the IGNYTE-ESO trial which explores lete-cel in the first-line setting for treatment naïve patients with metastatic or unresectable Synovial Sarcoma or MRCLS.
The company plans to launch lete-cel commercially in the US by 2026. This will be the second product in the sarcoma portfolio, targeting both Synovial Sarcoma and MRCLS, thereby significantly broadening treatment options for these cancers.
- AL3818 (anlotinib): Advenchen Laboratories
AL3818 is an orally administered receptor tyrosine kinase inhibitor targeting vascular endothelial growth factor receptors (VEGFR1, VEGFR2/KDR, and VEGFR3), stem cell factor receptor (C-kit), platelet-derived growth factor (PDGFβ), and fibroblast growth factor receptors (FGFR1, FGFR2, and FGFR3). AL3818 is being evaluated for the treatment of alveolar soft part sarcoma, leiomyosarcoma, and Synovial Sarcoma in a global Phase III trial. It is being developed by Advenchen Laboratories. The FDA and EMA granted AL3818 an orphan drug designation for the potential treatment of soft tissue sarcomas.
|
Comparison of Emerging Therapies | |||||
|
Product |
Company |
Phase |
Indication |
MoA |
RoA |
|
AL3818 |
Advenchen Laboratories |
III |
Synovial Sarcoma |
Tyrosine kinase inhibitor |
Oral |
|
Letetresgene autoleucel |
Adaptimmune Therapeutics |
II |
First-line setting for treatment naïve patients with metastatic or unresectable Synovial Sarcoma |
Autologous T-cell therapy |
Intravenous |
|
TBI-1301 |
Takara Bio |
I/II |
Synovial Sarcoma |
T-cell receptor (TCR) gene therapy |
Intravenous |
|
OTSA101 |
OncoTherapy Science |
I |
Refractory, relapsed, and/or advanced Synovial Sarcoma |
FZD10 antagonists |
Intravenous |
Detailed emerging therapies assessment will be provided in the final report.
Synovial Sarcoma Drugs Market Insights
- T-cell immunotherapy
T-cell therapy has indeed emerged as a promising treatment for Synovial Sarcoma. Specifically, the US Food and Drug Administration (FDA) has recently approved TECELRA (afamitresgene autoleucel), which is an autologous T-cell immunotherapy designed to target cancer cells expressing the MAGE-A4 antigen. It’s the first new treatment approved for Synovial Sarcoma in over a decade. This innovative therapy provides a critical option for adults with unresectable or metastatic Synovial Sarcoma who have received prior chemotherapy and meet specific criteria.
- FZD10 antagonists
FZD10 has garnered attention as a potential therapeutic target for Synovial Sarcoma. OTSA101 is an anti-FZD10 antibody and has been developed as novel cancer therapy for Synovial Sarcoma. OTSA-101 specifically delivers radiation to FZD10-expressing Synovial Sarcoma lesions. FZD10 is involved in the Wnt signaling pathway, which plays a crucial role in cancer development.
Synovial Sarcoma Market Outlook
Surgery remains the primary and only curative treatment for localized, resectable soft tissue sarcomas (STS), including Synovial Sarcoma, typically combined with radiotherapy. In terms of pharmacological therapies, which are the focus of market forecasts by DelveInsight, chemotherapy is the mainstay for metastatic sarcomas. For Synovial Sarcoma, the standard approach involves a fixed number of chemotherapy cycles with doxorubicin and ifosfamide as the first-line drugs, generating significant revenue in this field.
YONDELIS, an antitumor agent, is approved in Europe for advanced soft tissue sarcoma after failure of standard therapies or in patients unable to receive them. However, its effectiveness for Synovial Sarcoma in the US remains uncertain. VOTRIENT, an oral multi-target tyrosine kinase inhibitor, is currently the only TKI approved for various STS subtypes, including Synovial Sarcoma. It works by inhibiting VEGFR-mediated angiogenesis and blocking receptor tyrosine kinases involved in tumor growth.
Studies have shown that Synovial Sarcoma has multiple therapeutic targets, such as vascular endothelial growth factor, suggesting a need for new systemic treatments beyond traditional chemotherapy. In August 2024, the FDA granted accelerated approval to TECELRA (afamitresgene autoleucel), a T-cell therapy for unresectable or metastatic Synovial Sarcoma. This represents the first new treatment in over a decade, addressing a significant therapeutic gap and offering new hope for patients.
The Synovial Sarcoma Therapeutics Market Landscape is expected to change in the coming years owing to the launch of several upcoming therapies. A significant amount of research and developmental activities over the past several years have led to the gradual emergence of more effective and less toxic treatment modalities in Synovial Sarcoma patients. Some of the major players in clinical development are Advenchen Laboratories, Adaptimmune Therapeutics, Takara Bio, and others.
Key Findings
- In 2023, among the 7MM, the US has been the largest contributor and is expected to continue growing throughout the forecast period from 2024 to 2034.
- Among EU4 and the UK, Germany accounted for the maximum Synovial Sarcoma Market Size in 2023, while Spain occupied the bottom of the ladder.
- Surgery remains the cornerstone of treatment and the only curative locoregional approach to localized resectable STS including Synovial Sarcoma.
- Letetresgene autoleucel, a targeted therapy in development, is a NY-ESO-1-directed genetically modified autologous T-cell immunotherapy. With currently only one approved targeted therapy for Synovial Sarcoma, this new treatment is anticipated to secure a substantial portion of the market.
- Adaptimmune (ADAP) outlined its market rollout strategy, stating that it plans to establish at least 6–10 authorized treatment centers this year and expand to around 30 centers within the first two years.
Synovial Sarcoma Drugs Uptake
This section focuses on the uptake rate of potential Synovial Sarcoma drugs expected to be launched in the market during 2024–2034, which depends on the competitive landscape, safety, efficacy data, and order of entry. It is important to understand that the key players evaluating their novel therapies in the pivotal and confirmatory trials should remain vigilant when selecting appropriate comparators to stand the greatest chance of a positive opinion from regulatory bodies, leading to approval, smooth launch, and rapid uptake.
Synovial Sarcoma Pipeline Development Activities
The Synovial Sarcoma Therapeutics Market Report provides insights into therapeutic candidates in Phase III, Phase II, and Phase I. It also analyzes key Synovial Sarcoma Companies involved in developing targeted therapeutics. Synovial Sarcoma Companies like Advenchen Laboratories, Adaptimmune Therapeutics, and Takara Bio actively engage in late-stage and mid-stage research and development efforts for Synovial Sarcoma. The pipeline of Synovial Sarcoma possesses a potential drug, and there is a positive outlook for the therapeutics market, with expectations of growth during the forecast period (2024–2034).
Pipeline Development Activities
The Synovial Sarcoma Therapeutics Market Report covers information on collaborations, acquisitions and mergers, licensing, and patent details for Synovial Sarcoma emerging therapy.
Take Your Research to the Next Level! Click Here to Get Access to the Full Pipeline Report @ Synovial Sarcoma Treatment Drugs
KOL- Views
To keep up with current market trends, we take KOLs and SMEs’ opinions working in the domain through primary research to fill the data gaps and validate our secondary research. Industry Experts contacted for insights on the Synovial Sarcoma evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake, along with challenges related to accessibility, from experts including geneticists, pediatricians, orthopedists, endocrinologists, speech therapists, psychologists, dietitians, and nutritionists, and others.
DelveInsight’s analysts connected with 15+ KOLs to gather insights; however, interviews were conducted with 10+ KOLs in the 7MM. Universities such as the Department of Pathology, Derriford Hospital, University Hospitals Plymouth NHS Trust, Department of Orthopedic Surgery, Cancer Institute Hospital of the Japanese Foundation for Cancer Research, University of Washington, etc., were contacted. Their opinion helps understand and validate current and emerging therapy treatment patterns or Synovial Sarcoma market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
|
KOL Views |
|
“Synovial Sarcomas originating in the mediastinum are exceptionally rare and are often mistaken for other, more common neoplasms in this location, especially since there are no specific imaging characteristics or clinical manifestations. Contrary to Synovial Sarcomas of the extremities, mediastinal tumors more commonly affect male patients. Histologically, these tumors can be divided into monophasic, biphasic, and poorly differentiated variants, further complicating the diagnostic process. The treatment of mediastinal Synovial Sarcomas often requires multimodal therapy, including surgery, chemotherapy, and radiation. Despite this, the prognosis for Synovial Sarcomas in this location appears to be worse than for their analogs in the soft tissue, likely related to the often large size of the lesions and proximity to critical anatomic structures making complete surgical resection difficult to achieve.” – Department of Pathology, Derriford Hospital, University Hospitals Plymouth NHS Trust, Plymouth, UK |
|
“Sarcomas are heterogeneous and clinically challenging soft tissue and bone cancers. Although constituting only 1% of all human malignancies, sarcomas represent the second most common type of solid tumors in children and adolescents and comprise an important group of secondary malignancies. Current multimodal treatment concepts combine surgery, polychemotherapy (with/without local hyperthermia), irradiation, immunotherapy, and/or targeted therapeutics. Recent scientific advancements have enabled a more precise molecular characterization of sarcoma subtypes and revealed novel therapeutic targets and prognostic/predictive biomarkers.”
|
Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT analysis and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of gaps in disease diagnosis, patient awareness, physician acceptability, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided.
Conjoint Analysis analyzes multiple approved and emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy. In efficacy, the trial’s primary and secondary outcome measures are evaluated; for instance, in event-free survival, one of the most important primary outcome measures is event-free survival and overall survival.
Further, the therapies’ safety is evaluated wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Synovial Sarcoma Therapeutics Market Access and Reimbursement
Reimbursement may be referred to as the negotiation of a price between a manufacturer and payer that allows the manufacturer access to the market. It is provided to reduce the high costs and make the essential drugs affordable. Health technology assessment (HTA) plays an important role in reimbursement decision-making and recommending the use of a drug. These recommendations vary widely throughout the seven major markets, even for the same drug. In the US healthcare system, both Public and Private health insurance coverage are included. Also, Medicare and Medicaid are the largest government-funded programs in the US. The major healthcare programs including Medicare, Medicaid, Health Insurance Program (CHIP), and the state and federal health insurance marketplaces are overseen by the Centers for Medicare & amp; Medicaid Services (CMS). Other than these, Pharmacy Benefit Managers (PBMs), and third-party organizations that provide services, and educational programs to aid patients are also present.
The Synovial Sarcoma Therapeutics Market Report further provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of currently used therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
ASCO Highlights
|
Product |
Company |
Title |
Abstract |
Result |
|
ZEPZELCA (lurbinectedin) |
PharmaMar |
Efficacy and safety of lurbinectedin (LUR) with irinotecan (IRI) in a phase 2 expansion cohort of patients with Synovial Sarcoma. |
# 11560 |
The lurbinectedin/irinotecan combination showed activity in pretreated Synovial Sarcoma with manageable toxicity. These results reinforce the rationale for assessing the activity of lurbinectedin combined with irinotecan in Synovial Sarcoma and other tumor types. For irinotecan ORR: 19.2% Median DoR not reached Median PFS: 2.7 months |
|
Letetresgene autoleucel (lete-cel) |
Adaptimmune |
Lete-cel in patients with Synovial Sarcoma or myxoid/round cell liposarcoma: Planned interim analysis of the pivotal IGNYTE-ESO trial. |
# 2500 |
IGNYTE-ESO SS2 met the primary endpoint success criterion at this planned IA, with a 40% ORR consistent across SyS and MRCLS, and a known safety profile of hematologic toxicity and CRS. This supports the potential of lete-cel as a novel therapy for pts with advanced or metastatic SyS and MRCLS. Median duration of response: 10.6 months |
|
Innovent Biologics |
Sintilimab, doxorubicin and ifosfamide |
Sintilimab, doxorubicin, and ifosfamide (AI) as first-line treatment in patients with advanced undifferentiated pleomorphic sarcoma (UPS), Synovial Sarcoma (SS), myxoid liposarcoma (MLPS) and dedifferentiated liposarcoma (DDLPS): A single-arm phase 2 trial |
# 11505 |
ORR was 100% for Synovial Sarcoma. At a median follow-up time of 28.0 months, the median PFS and OS were 9.0 months and 19.9 months. The primary endpoint of ORR was met. This study indicated promising efficacy and safety of sintilimab combined with AI in the first-line treatment of Synovial Sarcoma. |
Synovial Sarcoma Therapeutics Market Report Scope
- The Synovial Sarcoma Therapeutics Market Report covers a segment of key events, an executive summary, and a descriptive overview, explaining its causes, signs, symptoms, pathogenesis, and currently used therapies.
- Comprehensive insight into the Synovial Sarcoma Epidemiology segments and forecasts, disease progression, and treatment guidelines has been provided.
- Additionally, an all-inclusive account of the marketed and emerging therapies and the elaborative profiles of late-stage and prominent therapies will impact the current treatment landscape.
- A detailed review of the Synovial Sarcoma Therapeutics Market, historical and forecasted Synovial Sarcoma Treatment Market Size, Synovial Sarcoma Drugs Market Share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The Synovial Sarcoma Therapeutics Market Report provides an edge while developing business strategies by understanding trends through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help shape and drive Synovial Sarcoma.
Synovial Sarcoma Therapeutics Market Report Insights
- Patient-based Synovial Sarcoma Market Forecasting
- Therapeutic Approaches
- Synovial Sarcoma Pipeline Drugs Analysis
- Synovial Sarcoma Market Size and Trends
- Existing and Future Synovial Sarcoma Drugs Market Opportunity
Synovial Sarcoma Therapeutics Market Report Key Strengths
- 11 Years Synovial Sarcoma Market Forecast
- The 7MM Coverage
- Synovial Sarcoma Epidemiology Segmentation
- Key Cross Competition
- Synovial Sarcoma Pipeline Drugs Uptake
- Key Synovial Sarcoma Market Forecast Assumptions
Synovial Sarcoma Therapeutics Market Report Assessment
- Current Synovial Sarcoma Treatment Market Practices
- Synovial Sarcoma Unmet Needs
- Synovial Sarcoma Pipeline Drugs Profiles
- Synovial Sarcoma Drugs Market Attractiveness
- Qualitative Analysis (SWOT and Analyst Views)
FAQs
- What was the Synovial Sarcoma market size, the market size by therapies, market share (%) distribution in 2023, and what would it look like by 2034? What are the contributing factors for this growth?
- What can be the future treatment paradigm for Synovial Sarcoma?
- What are the disease risks, burdens, and unmet needs of Synovial Sarcoma? What will be the growth opportunities across the 7MM concerning the patient population with Synovial Sarcoma?
- What are the current options for the Synovial Sarcoma Treatment? What are the current guidelines for treating Synovial Sarcoma in the 7MM?
- What are the recent novel therapies, targets, mechanisms of action, and technologies being developed to overcome the limitations of existing therapies?
Reasons to Buy
- The Synovial Sarcoma Therapeutics Market Report will help develop business strategies by understanding the latest trends and changing treatment dynamics driving Synovial Sarcoma.
- Insights on patient burden/disease Synovial Sarcoma Prevalence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years.
- Understand the existing Synovial Sarcoma Drugs Market opportunities in varying geographies and the growth potential over the coming years.
- Identifying strong upcoming players in the Synovial Sarcoma Drugs Market will help devise strategies to help get ahead of competitors.
- Detailed analysis ranking of class-wise potential therapies under the analyst view section to provide visibility around leading classes.
- Highlights of access and reimbursement policies of current therapies, barriers to accessibility of expensive off-label therapies, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing Synovial Sarcoma Drugs Market so that the upcoming players can strengthen their development and launch strategy.
Stay Updated with us for Recent Articles