Urethral Stricture Treatment Devices
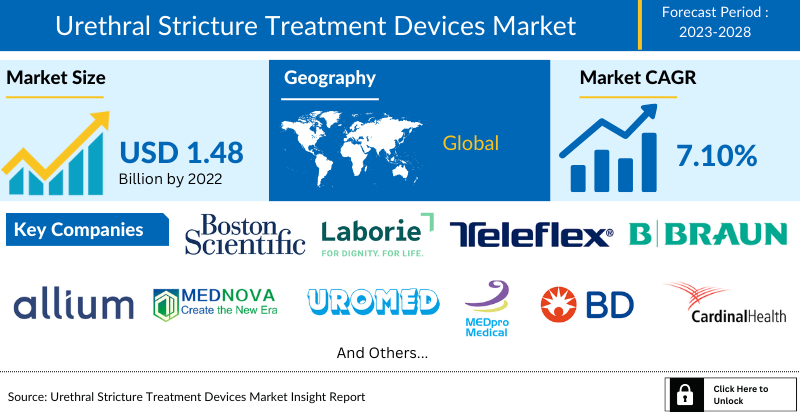
- The Global Urethral Stricture Treatment Devices Market has experienced substantial growth in recent years. According to research analysis, the Urethral Stricture Treatment Devices market size was valued at over ~1.48 Billion in 2023, and it is expected to continue growing at a Compound Annual Growth Rate (CAGR) of around 7.10% from 2024 to 2030.
- The leading companies working in the Urethral Stricture Treatment Devices Market include Boston Scientific Corporation, Laborie, Cook, Teleflex Incorporated, B. Braun Melsungen AG, Allium Ltd., Pnn Medical A/S, Zhejiang ChuangXiang Medical Technology Co., LTH, UROMED Kurt Drews KG, Med pro-Medical B.V., BD, Cardinal Health. Coloplast, ConvaTec Inc, BACTIGUARD AB, and others.
Download the Sample PDF to Get More Insight @ Urethral Stricture Treatment Devices Market
Urethral Stricture Treatment Devices Market By Product Type (Urethral Dilators, Urethral Stents, Urethral Balloon Dilation Catheters, and Urinary Catheters), Stricture Location (Anterior and Posterior), End-User (Hospitals, Specialty Clinics, and Others), and Geography is expected to grow at a steady CAGR forecast till 2030) owing to increase in the probability of urethral stricture formation as a complication of cancer treatment for prostate and colorectal cancers as well as extensive use of urinary catheters
The global urethral stricture treatment devices market was valued at ~USD 1.48 billion in 2023, growing at a CAGR of 7.10% during the forecast period from 2024 to 2030 to reach USD 2.23 billion by 2030. The demand for urethral stricture treatment devices is witnessing a surge majorly due to the rising prevalence of cancers in the pelvic region such as colorectal and prostate cancers wherein the cancer treatment is linked with an increased probability of urethral stricture formation, growing prevalence of benign prostatic hyperplasia in which urethral stricture may form as a complication of surgical interventions, increasing geriatric population wherein aging plays a major role in the development of numerous diseases such as neurogenic bladder leading to the development of urethral strictures due to extensive use of urinary catheters, and advancements in product development such as drug-eluting urology balloon thereby boosting the growth of the urethral stricture treatment devices market during the forecast period.
|
Study Period |
2021 to 2030 |
|
Forecast Period |
2024 to 2030 |
|
Geographies Covered |
Global |
|
Urethral Stricture Treatment Devices CAGR | 7.10% |
|
Urethral Stricture Treatment Devices Market Size |
~1.48 Billion in 2023 |
|
Urethral Stricture Treatment Devices Companies |
|
Urethral Stricture Treatment Devices Market Dynamics
The urethral stricture treatment devices market is observing a surge in product demand due to a plethora of reasons one of them being the rising prevalence of various cancers specifically in the pelvic region such as colorectal and prostate cancers. As per the data cited by the World Health Organization (WHO), in 2020, cancer of the colon and rectum was the third-most common cancer type globally and accounted for 1.93 million new cases. In the same factsheet by the WHO, in 2020, prostate cancer was the fourth-most prevalent cancer type globally and accounted for 1.41 million new cases.
Urethral strictures have been associated as a complication of radiation treatment for prostate and rectal cancers, surgery for an enlarged prostate or prostate cancer, or trauma to the genitals or perineum. It has been established in various studies that radiation therapy for cancer treatment, particularly prostate cancer, leads to reduced vascularity around the treatment area. Furthermore, the risk of developing urethral strictures appears to enhance further in a patient receiving both brachytherapy and external beam radiation therapy. Additionally, it has also been reported that radial prostatectomy also significantly increases the chances of the formation of urethral strictures thereby indicating that treatment of cancers in the pelvic region may result in the formation of urethral strictures.
Therefore, the rising prevalence of cancers in the pelvic region may result in an increased incidence of urethral strictures which in turn may drive the demand for urethral strictures treatment devices ultimately driving the urethral strictures treatment devices market during the forecast period. Another important aspect driving the demand for the urethral stricture treatment devices market is the rising geriatric population. As per the statistics quoted by the World Health Organization in their Decade of Healthy Ageing (2021-2030) Report, it has been estimated that people in the age bracket of 60 years and older will account for 34% more share in the total population, increasing from one billion in 2019 to 1.4 billion by 2030.
The report further mentioned that by 2050, the global elderly population is expected to reach 2.1 billion. Aging is considered one of the key risk factors that heavily contributes to the development of various indications such as benign prostatic hyperplasia (BPH), and neurogenic bladder among other indications. BPH is most commonly treated by simple prostatectomies and transurethral resection of the prostate wherein urethral strictures are reported as a common complication. Furthermore, the increasing prevalence of neurogenic bladder also results in increased chances of urethral stricture formation due to trauma or injury caused by the extensive use of urinary catheters.
Therefore, the rising geriatric population is expected to result in the increasing prevalence of diseases such as BPH where the treatment may result in the formation of urethral strictures, thereby bolstering the growth of urethral stricture treatment devices in coming years. However, device-related complications and the availability of alternative surgical approaches such as urethroplasty may be limiting factors for the growth of the urethral stricture treatment devices market.
Urethral Stricture Treatment Devices Market Segment Analysis
Urethral Stricture Treatment Devices Market by Product Type (Urethral Dilators, Urethral Stents, Urethral Balloon Dilation Catheters, and Urinary Catheters), Stricture Location (Anterior and Posterior), End-User (Hospitals, Specialty Clinics, and Others), and Geography (North America, Europe, Asia-Pacific, and Rest of the World).
In the product segment of the urethral stricture treatment devices market, the urethral balloon dilation catheters category is expected to register a healthy CAGR in the urethral stricture treatment devices market during the forecast period. This can be attributed to the advantages offered by these devices over their counterparts. Balloon dilation is a well-tolerated, safe, office-based procedure that offers multiple advantages over sequential rigid dilation and internal urethrotomy. It is associated with minimal complications. Furthermore, urethral balloon dilation is considered a minimally invasive technique that has the potential advantage of being less morbid and is technically less challenging to perform. It is also considered to be less traumatic than sequential rigid dilatation.
Owing to the advantages associated with urethral balloon dilation catheters, there has been an increase in the development of newer products aimed at treating urethral strictures. For instance, in December 2021, Urotronic received regulatory approval from the US Food and Drug Administration for their product- Optilume, a drug-coated urology balloon, which elutes a highly lipophilic drug, paclitaxel that limits hyperactive cell proliferation and the fibrotic scar tissue generation that results in stricture recurrence.
Therefore, the advantages associated with urethral balloon dilation and the innovation in product development are expected to boost the demand for this product category thereby ultimately propelling the market for urethral balloon dilation catheters during the forecast period. In italy and switzerland urethral strictures market by type is also expected tp show a positive growth in the market.
North America is expected to dominate the overall Urethral Stricture Treatment Devices Market
Among all the regions, North America is estimated to account for the largest share of the urethral stricture treatment devices market. This domination is due to the increase in the prevalence of sexually transmitted diseases, and the increasing prevalence of cancers in the region. Furthermore, the rising geriatric population as well as growing cases of obesity in the US population are also expected to result in a growing number of urethral strictures in the North American region. Additionally, extensive research and development activities about product development and a conducive regulatory environment further help product manufacturers to generate more revenue from the region.
One of the key driving factors of the United States urethral stricture treatment devices market is the interplay of various factors in the country. For instance, as per the data cited by the Centers for Disease Control and Prevention (2023), in 2019, 2.6 million cases of sexually transmitted diseases were reported in the country registering a 6th–consecutive year increase from 1.4 million in 2014. STDs like gonorrhea are reported to cause infection and inflammation in the urethra, leading to scarring and stricture. Some chronic inflammatory skin conditions like lichen sclerosis can also cause urethral strictures. Therefore, the presence of a large patient population suffering from sexually transmitted diseases correlates to the presence of a potential patient population that may resort to using urethral stricture devices as a treatment method, thus driving the product demand in the nation.
Moreover, the increasing obese population is also expected to drive the demand for urethral stricture treatment devices in the country. For instance, as per the data provided by the CDC (2023), in 2017-2018, the obesity prevalence in the country was 42.4%. Obesity has been associated with increased mean urethral stricture length in obese patients compared to patients with a normal body-mass index.
Thus, the interplay of all the aforementioned factors is likely to fuel the market growth of urethral stricture treatment devices in the United States during the forecast period.
Urethral Stricture Treatment Devices Companies
Some of the key market players operating in the urethral stricture treatment devices market include Boston Scientific Corporation, Laborie, Cook, Teleflex Incorporated, B. Braun Melsungen AG, Allium Ltd., Pnn Medical A/S, Zhejiang ChuangXiang Medical Technology Co., LTH, UROMED Kurt Drews KG, Med pro-Medical B.V., BD, Cardinal Health. Coloplast, ConvaTec Inc, BACTIGUARD AB, and others.
Recent Developmental Activities in the Urethral Stricture Treatment Devices Market:
- In December 2021, Urotronic received regulatory approval from the US Food and Drug Administration for their product- Optilume, a drug-coated urology balloon, which elutes a highly lipophilic drug, paclitaxel that limits hyperactive cell proliferation and the fibrotic scar tissue generation that results in stricture recurrence.
Key Takeaways from the Urethral Stricture Treatment Devices Market Report Study
- Market size analysis for current urethral stricture treatment devices market size (2023), and market forecast for 5 years (2024-2030)
- Top key product/services/technology developments, mergers, acquisitions, partnerships, and joint ventures happened for the last 3 years
- Key companies dominating the global urethral stricture treatment devices market.
- Various opportunities available for the other competitor in the urethral stricture treatment devices market space.
- What are the top-performing segments in 2023? How these segments will perform in 2030?
- Which are the top-performing regions and countries in the current urethral stricture treatment devices market scenario?
- Which are the regions and countries where companies should have concentrated on opportunities for urethral stricture treatment devices market growth in the coming future?
Target Audience who can be benefited from this Urethral Stricture Treatment Devices Market Report Study
- Urethral stricture treatment devices products providers
- Research organizations and consulting companies
- Urethral stricture treatment device-related organizations, associations, forums, and other alliances
- Government and corporate offices
- Start-up companies, venture capitalists, and private equity firms
- Distributors and Traders dealing in urethral stricture treatment devices
- Various End-users who want to know more about the urethral stricture treatment devices market and the latest technological developments in the urethral stricture treatment devices market.
Also Read @ Latest DelveInsight Blogs