Warm Autoimmune Hemolytic Anemia Market Summary
- The Warm Autoimmune Hemolytic Anemia market in the 7MM is projected to grow at a significant CAGR by 2034 in the leading countries (US, EU4, UK and Japan).
Warm Autoimmune Hemolytic Anemia Market and Epidemiology Analysis
- In 2023, in the 7MM the total wAIHA market size was ~ USD 300 million.
- wAIHA is the most common type of autoimmune hemolytic anemia, comprising nearly 70–80% of all adult cases and 50% of pediatric cases. wAIHA can develop at any age, but the median age of onset is 52 years.
- The primary and widely used treatment for wAIHA involves corticosteroids, which serve as the fundamental approach. If corticosteroids prove ineffective, the possibility of undergoing a splenectomy emerges as an alternative. Rituximab is now considered a viable option for cases that resist conventional treatments, while the employment of immunosuppressive medications can offer assistance, particularly in managing chronic severe refractory instances.
- Additionally, immunosuppressive agents like azathioprine, cyclophosphamide, and mycophenolate mofetil have emerged as valuable adjuncts, particularly in cases of chronic severe refractory AIHA.
- In refractory or recurrent cases, sequential treatment lines are used. AIHA is still a disease that causes difficulties in treatment and this is why it is important to develop research into new drugs in AIHA.
- Innovative targeted therapies that address the underlying pathogenesis of wAIHA are needed, as there is currently no approved treatment for this disease. Promising novel treatments for wAIHA include new B-cell/plasma-cell targeting agents, complement inhibitors, and agents removing the pathogenic autoantibodies via neonatal Fc receptor blocking.
- Novel agents such as Ianalumab, Nipocalimab, Rilzabrutinib, and Povetacicept will increase the therapeutic armamentarium and possibly fill the gap of wAIHA relapsed after/refractory to rituximab. Moreover, these new target therapies may represent a tool for the unmet needs of very acute cases.
- Since there are now no licensed treatments for wAIHA, the market opportunity is quite attractive. The current standard of care is limited by drugs' waning effectiveness over time and their adverse effect profiles, which increase the burden of symptoms for patients. If new medications are able to gain this indication, they might potentially grab a valuable market due to the lack of competition and unmet demand for a drug.
- Rituximab continues to be the leader in the wAIHA competitive landscape in terms of both efficacy and familiarity.
- In the landscape of wAIHA treatment, rilzabrutinib, developed by Novartis/MorphoSys, stands out as a potent oral BTK inhibitor tailored for immune-mediated diseases.
|
Study Period |
2020 to 2034 |
|
Forecast Period |
2026-2034 |
|
Geographies Covered |
|
|
wAIHA Market |
|
|
wAIHA Market Size | |
|
wAIHA Companies |
|
Factors Impacting the Warm Autoimmune Hemolytic Anemia Market Growth
-
Development of novel targeted biologics and immunomodulators
Next-generation therapies including FcRn inhibitors, Syk inhibitors, kinase inhibitors, and monoclonal antibodies are transforming the autoimmune hemolysis treatment landscape, offering higher success rates in clinical trials with improved efficacy and fewer side effects compared to traditional immunosuppressants
-
Growing prevalence of autoimmune disorders globally
Rising cases of autoimmune diseases in developed and emerging regions are expanding the patient population requiring treatment, with warm autoimmune hemolytic anemia representing the most common subtype and benefiting from higher prevalence compared to cold agglutinin disease
-
Accelerated regulatory pathways and orphan drug designations
FDA Fast Track and Orphan Drug Designation programs are expediting approval of innovative medicines for this rare hematological disorder, with favorable orphan drug policies supporting faster market entry and encouraging pharmaceutical investment
-
Advancement in diagnostic methodologies and disease awareness
Improved diagnostic criteria, enhanced physician awareness, and sophisticated diagnostic instruments are enabling earlier disease identification and treatment initiation, with growing patient advocacy efforts supporting increased diagnosis rates
-
Expanding healthcare infrastructure and distribution channels
Strong presence of pharmaceutical players, advanced healthcare systems particularly in North America, and evolving distribution landscapes including hospital pharmacies for biologic infusions and digital pharmacy platforms are improving treatment accessibility and market penetration
-
Robust R&D investments and clinical trial activity
Pharmaceutical companies and research institutions are actively engaged in developing advanced therapeutic solutions with ongoing research into disease mechanisms expanding treatment options, while strong clinical trial activity and research funding are sustaining market growth momentum
DelveInsight’s "Warm Autoimmune Hemolytic Anemia (wAIHA) Market Insights, Epidemiology, and Market Forecast – 2034" report delivers an in-depth understanding of wAIHA, historical and forecasted epidemiology as well as the wAIHA market trends in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
The wAIHA market report provides current treatment practices, emerging drugs, wAIHA market share of individual therapies, and current and forecasted wAIHA market size from 2020 to 2034, segmented by seven major markets. The Warm Autoimmune Hemolytic Anemia therapeutics market report also covers current wAIHA treatment practices/algorithms and unmet medical needs to curate the best of the opportunities and assess the underlying potential of the Warm Autoimmune Hemolytic Anemia treatment market.
Warm Autoimmune Hemolytic Anemia (wAIHA) Treatment Market
wAIHA Overview
Warm autoimmune hemolytic anemia (wAIHA) is a type of autoimmune disorder characterized by the destruction of red blood cells (hemolysis) due to the body's immune system mistakenly targeting its red blood cells. In wAIHA, the antibodies produced by the immune system bind to red blood cells at body temperature (hence "warm") and mark them for destruction by the spleen and other organs. This leads to a decrease in the number of circulating red blood cells, resulting in anemia. Symptoms of wAIHA can vary but often include fatigue, weakness, pallor, shortness of breath, and jaundice (yellowing of the skin and eyes). The condition can be primary (idiopathic) or secondary to underlying conditions such as autoimmune diseases, infections, lymphoproliferative disorders, or certain medications. Diagnosis involves blood tests, and treatment typically includes immunosuppressive medications to suppress the abnormal immune response and alleviate symptoms.
wAIHA Diagnosis
Diagnosing wAIHA involves several steps to confirm the presence of hemolysis (destruction of red blood cells) and to identify the underlying cause. Initially, a complete blood count (CBC) is conducted to assess hemoglobin levels, red blood cell count, and other parameters indicative of anemia. Peripheral blood smear examination may reveal signs of hemolysis, such as red blood cell destruction and the presence of spherocytes (abnormally shaped red blood cells). Direct antiglobulin test (DAT), also known as the Coombs test, is performed to detect antibodies or complement proteins bound to the surface of red blood cells. A positive DAT result confirms the presence of autoimmune-mediated hemolysis. Further tests, including serum protein electrophoresis, autoimmune serology, and bone marrow examination, may be conducted to identify underlying conditions associated with wAIHA, such as autoimmune diseases, infections, or malignancies. Overall, a combination of clinical findings and laboratory tests is crucial for the accurate diagnosis of wAIHA and for determining appropriate treatment strategies.
Further details related to diagnosis will be provided in the report…
wAIHA Treatment
The treatment of warm autoimmune hemolytic anemia (wAIHA) aims to suppress the autoimmune response, alleviate symptoms, and manage complications. Corticosteroids, such as prednisone, are often the first-line therapy, as they help suppress the immune system's abnormal activity and reduce red blood cell destruction. In cases where corticosteroids are ineffective or poorly tolerated, other immunosuppressive medications may be used, including azathioprine, rituximab, mycophenolate mofetil, or cyclophosphamide. Intravenous immunoglobulin (IVIG) therapy may also be considered for rapid symptom relief in severe cases. In some instances, splenectomy (surgical removal of the spleen) may be recommended, particularly if other treatments fail or are contraindicated. However, splenectomy carries risks and is generally reserved for refractory cases. Additionally, supportive care measures, such as blood transfusions to alleviate severe anemia and folic acid supplementation to support red blood cell production, may be necessary. Long-term management involves monitoring for disease activity, adjusting treatment as needed, and addressing any underlying conditions contributing to wAIHA. Collaboration with hematologists and rheumatologists is essential to develop individualized treatment plans and optimize outcomes for patients with wAIHA.
Further details related to treatment will be provided in the report…..
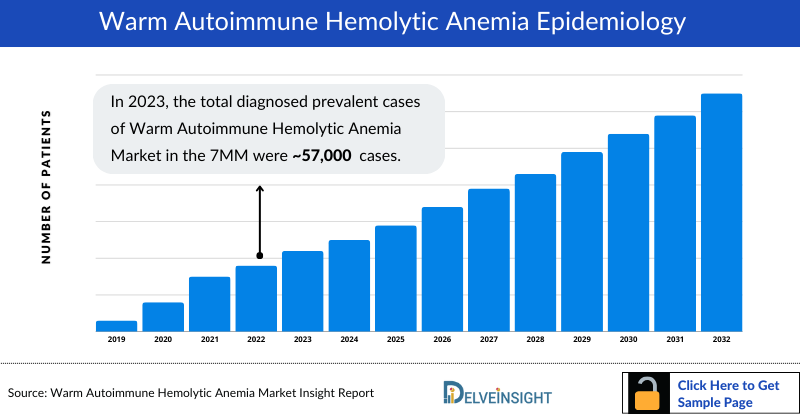
Warm Autoimmune Hemolytic Anemia (wAIHA) Epidemiology
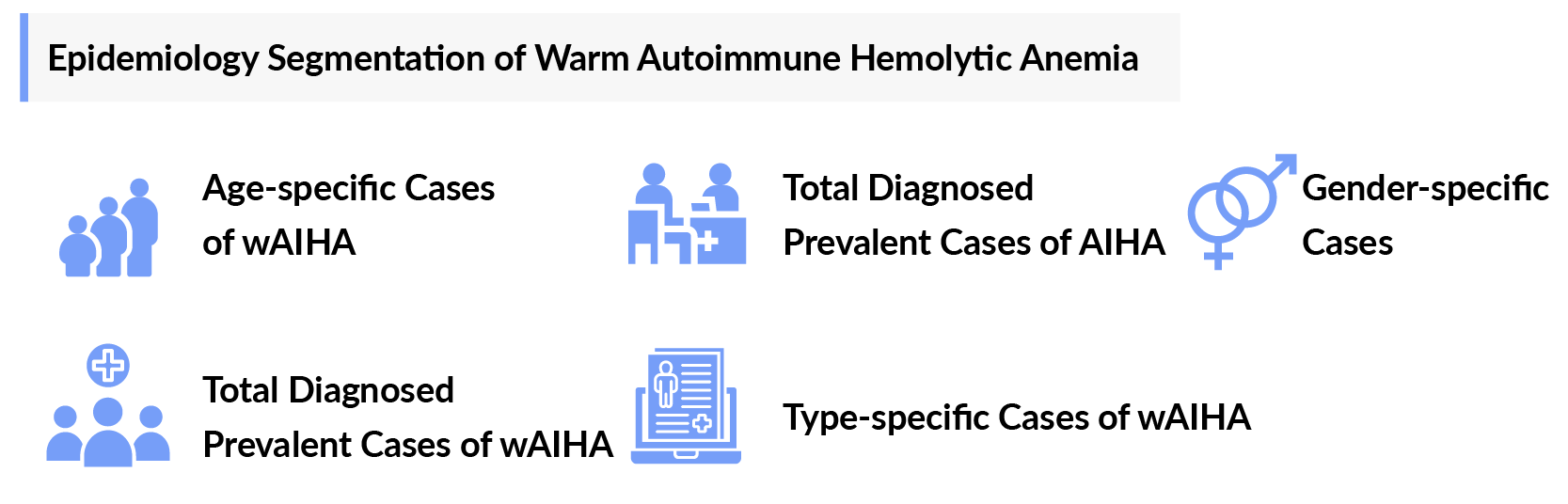
The wAIHA epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by the total diagnosed prevalent cases of autoimmune hemolytic anemia, total prevalent cases of wAIHA, type-specific cases of wAIHA, gender-specific cases of wAIHA, and age-specific cases of wAIHA in the 7MM market covering the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan from 2020 to 2034.
Key Findings from the Warm Autoimmune Hemolytic Anemia (wAIHA) Epidemiology
- Among the 7MM, the US accounted for the highest prevalent cases of autoimmune hemolytic anemia in 2023, with around 57,000 cases; these cases are expected to increase during the forecast period.
- Among gender-specific prevalent cases of wAIHA, females stand out as major contributors. In 2023, Females accounted for up to 60% cases of wAIHA. These cases are anticipated to increase by 2034 in the US.
- Amongst EU4 and the UK, the total prevalent cases of wAIHA were highest in Germany, while the lowest number of cases were in Spain in 2023.
- According to the estimates, in Japan, it is observed that wAIHA was most prevalent in the =65 year’s age group, accounting for over 64% of total cases in 2023.
Warm Autoimmune Hemolytic Anemia (wAIHA) Epidemiology Segmentation
- Total diagnosed prevalent cases of autoimmune hemolytic anemia
- Total prevalent cases of wAIHA
- Type-specific cases of wAIHA
- Gender-specific cases of wAIHA
- Age-specific cases of wAIHA
Warm Autoimmune Hemolytic Anemia (wAIHA) Drug Analysis
The drug chapter segment of the Warm Autoimmune Hemolytic Anemia treatment market report encloses a detailed analysis of the late-stage (Phase III and Phase II/III) and early-stage (Phase I/II) pipeline drugs. The current key Warm Autoimmune Hemolytic Anemia companies for emerging drugs and their respective drug candidates include Zenas BioPharma (Obexelimab) and Novartis/MorphoSys (Ianalumab). The drug chapter also helps understand the wAIHA clinical trial details, expressive pharmacological action, agreements and collaborations, approval, and patent details, and the latest news and press releases.
Emerging wAIHA Drugs
Obexelimab (ZB012): Zenas BioPharma
Obexelimab is a novel bifunctional antibody with first-in-class potential that inhibits B-cell lineages that express CD19. Simultaneous binding to CD19 and Fc?RIIB by obexelimab mimics a naturalantigen–antibody complex and downregulates B-cell activity. In early-stage clinical studies, obexelimab effectively demonstrated inhibition of B-cell function without depleting the cells and generated an encouraging treatment effect in patients with multiple autoimmune diseases. Currently, the drug is being evaluated in Phase III (SApHiAre trial) clinical stage of development.
In September 2023, Zenas BioPharma entered into a license and collaboration agreement with Bristol Myers Squibb to develop and commercialize obexelimab for autoimmune diseases in Japan, South Korea, Taiwan, Singapore, Hong Kong, and Australia.
Nipocalimab (M281): Johnson & Johnson Innovative Medicine
Nipocalimab is a fully human monoclonal antibody that targets the neonatal crystallizable fragment receptor (FcRn) with potential immunomodulating activity. Upon administration, nipocalimab targets and binds to FcRn at the IgG binding site, thereby preventing the interaction between FcRn and the serum protein IgG. By preventing FcRn/IgG binding, nipocalimab blocks the FcRn-mediated rescue of IgG, enables IgG degradation, and prevents IgG-mediated inflammation. Nipocalimab was granted FTD for wAIHA in July 2019 and ODD in December 2019. Currently, the drug is being evaluated in Phase II/III (NCT04119050) clinical stage of development.
|
Phase |
MoA |
|
III |
Binding to both CD19 and FcγRIIB |
|
III |
Anti-BAFF-R |
|
II/III |
Targeting and binding to FcRn at the IgG binding site |
|
I/II |
Inhibition of BAFF, BLyS |
wAIHA Drug Class Insight
BTK inhibitor
BTK plays a crucial role in B-cell receptor signaling, which is involved in the activation and proliferation of B cells. By inhibiting BTK, these medications can modulate B-cell function and the immune response, potentially dampening the autoimmune reaction responsible for wAIHA. Rilzabrutinib was an investigational drug, and there was limited information available about its use specifically for the treatment of warm autoimmune hemolytic anemia (wAIHA). However, given its mechanism of action as a Bruton's tyrosine kinase (BTK) inhibitor, similar to other drugs in this class like ibrutinib, there is potential for rilzabrutinib to be explored as a treatment option for wAIHA.
Warm Autoimmune Hemolytic Anemia (wAIHA) Market Outlook
The most common therapy and the cornerstone of treatment for Warm Autoimmune Hemolytic Anemia are corticosteroids. If these are ineffective, a splenectomy can be considered. Rituximab has become an option in refractory disease, and the use of immunosuppressors can be helpful in chronic severe refractory cases.
While glucocorticoids are considered the first-line treatment in WAIHA, this was empirically derived. Mechanisms of actions include suppression of autoantibody production, reduction in autoantibody affinity, and decreased destruction of erythrocytes by splenic macrophages, perhaps by diminished expression of Fc? receptors. Rituximab is used in diagnosed severe cases or in cases where long-term corticosteroids should be avoided. Although rituximab (an anti-CD20 antibody) is considered a second-line treatment, the combination of rituximab and prednisone at relatively low doses (100 mg once weekly, four times) is increasingly becoming a first-line treatment. With the advent of rituximab, azathioprine, cyclophosphamide, cyclosporine, and intravenous immunoglobulin became second or third-line treatments.
Conventional immunosuppressive drugs (such as azathioprine, cyclophosphamide, and cyclosporine), although widely used in clinical practice mainly as steroid-sparing agents, are moving to later lines.
Rilzabrutinib, a reversible, covalent Bruton tyrosine kinase (BTK) inhibitor, is also being studied in a Phase IIb trial. This drug also inhibits phagocytosis via interaction with the syk pathway. Several other therapies, including Obexelimab (ZB012), Ianalumab, and Povetacicept, are expected to fill the treatment gap and fulfill the current unmet needs
Key Findings from the Warm Autoimmune Hemolytic Anemia (wAIHA) Market Analysis
- The total wAIHA market size in the US was estimated to be ~USD 200 million in 2023, which is expected to grow during the forecast period (2024–2034).
- In EU4 and the UK, Germany accounted for the largest wAIHA market share with USD 20 million market size in 2023, which is expected to grow during the forecast period (2024–2034).
- In 2034, among the emerging Warm Autoimmune Hemolytic Anemia therapies, the highest wAIHA revenue was generated by Nipocalimab (M281), in Japan.
- Conventional immunosuppressive drugs (such as azathioprine, cyclophosphamide, and cyclosporine), although widely used in clinical practice mainly as steroid-sparing agents, are moving to later lines.
Warm Autoimmune Hemolytic Anemia (wAIHA) Drugs Uptake
This section focuses on the uptake rate of potential wAIHA drugs expected to be launched in the wAIHA market during 2024–2034. The landscape of wAIHA treatment has experienced a transformation with the uptake of novel drugs. These innovative therapies are redefining standards of care. Furthermore, the increased uptake of these transformative drugs is a testament to the unwavering dedication of hematologists, Researchers, professionals, and the entire healthcare community in their tireless pursuit of advancing eye care. This momentous shift in treatment paradigms is a testament to the power of research, collaboration, and human resilience.
Warm Autoimmune Hemolytic Anemia (wAIHA) Clinical Trials Analysis
The wAIHA market report provides insights into wAIHA clinicla trials within Phase III, and Phase II. It also analyzes key Warm Autoimmune Hemolytic Anemia companies involved in developing targeted therapeutics. wAIHA Companies like Zenas BioPharma and Novartis/MorphoSys actively engage in late-stage research and development efforts for wAIHA. The pipeline of wAIHA possesses a few potential drugs. However, there is a positive outlook for the Warm Autoimmune Hemolytic Anemia therapeutics market, with expectations of growth during the forecast period (2024–2034).
Pipeline Development Activities
The wAIHA drugs market report covers information on collaborations, acquisitions and mergers, licensing, and patent details for wAIHA emerging therapy.
Latest KOL Views on the Warm Autoimmune Hemolytic Anemia (wAIHA)
To keep up with current wAIHA market trends, we take KOLs and SMEs’ opinions working in the domain through primary research to fill the data gaps and validate our secondary research. Industry Experts contacted for insights on the wAIHA evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake, along with challenges related to accessibility, including Hematologist, Rheumatologist, Hematology-Oncology Specialist, and others.
DelveInsight’s analysts connected with 15+ KOLs to gather insights; however, interviews were conducted with 5+ KOLs in the 7MM. Centers such as the University of California Los Angeles Medical Center, Professor of Massachusetts General Hospital, MD, Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS), etc., were contacted. Their opinion helps understand and validate current and emerging therapy treatment patterns or wAIHA market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
wAIHA Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT analysis and Analyst views. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the Analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving treatment landscape.
Warm Autoimmune Hemolytic Anemia Market Access and Reimbursement
wAIHA is characterized by evidence of red blood cell (RBC) hemolysis and a direct antiglobulin test positive for IgG and sometimes complement. While varying with the extent of the compensatory increase in RBC production, symptoms of anemia predominate, as does jaundice, the latter often exacerbated by concurrent Gilbert’s syndrome. Initial treatment with corticosteroids is highly effective, with over 85% of patients responding but with less than one-third maintaining that response upon weaning. Subsequent rituximab administration in those failing corticosteroids provides complete remission in over 75% of patients and may be long-lasting. Over 50% of patients failing rituximab respond to erythropoiesis-stimulating agents or immunosuppressive agents. Splenectomy is best deferred if possible but does offer long-term remission in over two-thirds of patients. Current treatment approaches include corticosteroids, rituximab, immunosuppressive drugs, and splenectomy, but there are, so far, no disease-targeted therapies for wAIHA.
Rituximab: NICE
Rituximab is not licensed for treating autoimmune hemolytic anemia, so use for this indication is off-label. Rituximab 10 mg/mL concentrate for solution for intravenous infusion.
Comparing the cost of rituximab with other therapies for autoimmune hemolytic anemia is difficult because there is a lack of evidence to confirm the optimal dose, guide the use of recurrent courses in refractory cases, and confirm the advice on other aspects of the clinical pathway, such as combination with other treatments.
ICD-10 code D59.11 for wAIHA is a medical classification as listed by WHO under the range of diseases of the blood and blood-forming organs and certain disorders involving the immune mechanism.
Detailed market access and reimbursement assessment will be provided in the final report.
Scope of the wAIHA Market Report
- The wAIHA market report covers a segment of key events, an executive summary, and a descriptive overview of wAIHA, explaining its causes, signs, symptoms, pathogenesis, and currently used therapies.
- Comprehensive insight into the epidemiology segments and forecasts, disease progression, and treatment guidelines has been provided.
- Additionally, an all-inclusive account of the emerging therapies and the elaborative profiles of late-stage and prominent therapies will impact the current treatment landscape.
- A detailed review of the wAIHA market, historical and forecasted market size, market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The wAIHA market report provides an edge while developing business strategies by understanding trends through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help shape and drive wAIHA.
wAIHA Market Report Insights
- wAIHA Patient Population
- wAIHA Therapeutic Approaches
- wAIHA Pipeline Analysis
- wAIHA Market Size
- wAIHA Market Trends
- Existing and Future wAIHA Market Opportunity
wAIHA Market Report Key Strengths
- Eleven Years Forecast
- The 7MM Coverage
- wAIHA Epidemiology Segmentation
- Key Cross Competition
- wAIHA Drugs Uptake
- Key wAIHA Market Forecast Assumptions
wAIHA Market Report Assessment
- Current Treatment Practices
- wAIHA Unmet Needs
- wAIHA Pipeline Product Profiles
- wAIHA Market Attractiveness
- Qualitative Analysis (SWOT and Analyst Views)
- wAIHA Market Drivers
- wAIHA Market Barriers
Key Questions Answered in the wAIHA Market Report
- What was the wAIHA market size, the market size by therapies, market share (%) distribution in 2020, and what would it look like by 2034? What are the contributing factors for this growth?
- What can be the future treatment paradigm for wAIHA?
- What are the disease risks, burdens, and unmet needs of wAIHA? What will be the growth opportunities across the 7MM concerning the patient population with wAIHA?
- What are the current options for the treatment of wAIHA? What are the current guidelines for treating wAIHA in the 7MM?
- What are the recent novel therapies, targets, mechanisms of action, and technologies being developed to overcome the limitations of existing therapies?
- What is the patient share in wAIHA market?
Reasons to Buy wAIHA Market Report
- The wAIHA market report will help develop business strategies by understanding the latest trends and changing treatment dynamics driving wAIHA.
- Insights on patient burden/Warm Autoimmune Hemolytic Anemia prevalence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years.
- Understand the existing market opportunities in varying geographies and the growth potential over the coming years.
- Identifying strong upcoming Warm Autoimmune Hemolytic Anemia companies in the market will help devise strategies to help get ahead of competitors.
- Detailed analysis ranking of class-wise potential current and emerging therapies under the analyst view section to provide visibility around leading classes.
- Highlights of access and reimbursement policies of current therapies, barriers to accessibility of expensive off-label therapies, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing waiha market so that the upcoming Warm Autoimmune Hemolytic Anemia companies can strengthen their development and launch strategy.

-01.png)
-02.png)



