BTK Inhibitors Market
- According to DelveInsight, BTK Inhibitor market is expected to grow at a decent CAGR by 2034
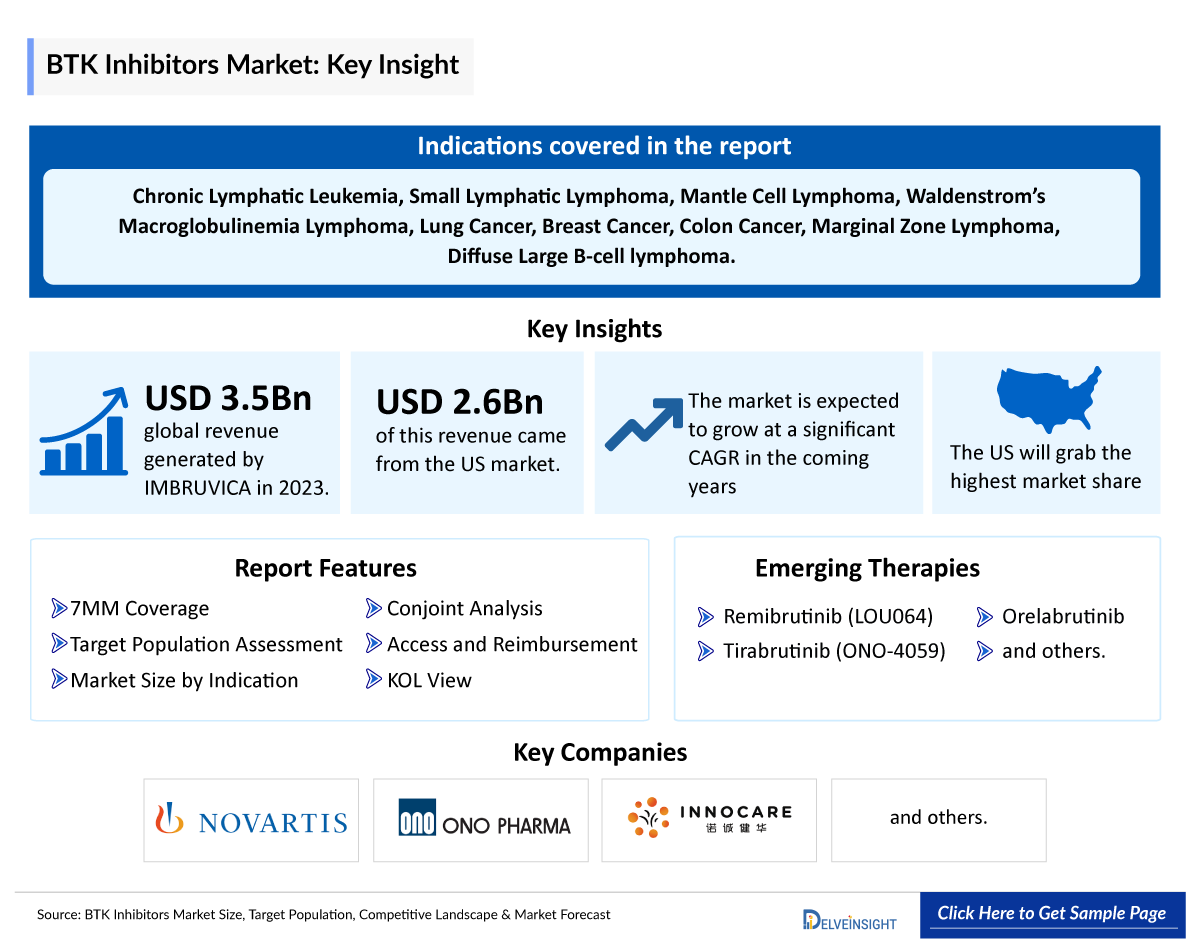
- In 2013, IMBRUVICA (ibrutinib), a BTK inhibitor got approval from the US FDA BTK Inhibitor Market to treat patients with Mantle Cell Lymphoma (MCL). Then again, in 2014, the US FDA expanded its use for Chronic Lymphocytic Leukemia (CLL) patients who have received at least one previous therapy. Additionally, ibrutinib was also later approved for the treatment of Waldenström’s Macroglobulinemia, Chronic Graft versus Host Disease along with above mentioned disease.
- IMBRUVICA generated approximately USD 3.5 billion in revenue globally and USD 2.6 billion in the US in 2023.
- Various other BTK inhibitors, including acalabrutinib, zanubrutinib, pirtobrutinib have received approval from regulatory bodies such as the Food and Drug Administration (FDA) and European Medicine Agency (EMA).
- Furthermore, numerous BTK inhibitors are presently undergoing clinical trials. Examples include Merck’s remibrutinib, tirabrutinib, and orelabrutinib. These drugs are currently in the development phase and are expected to receive regulatory approval during the forecast period.
- BTK inhibitors work by blocking the activity of the BTK enzyme and inhibiting the abnormal growth of B-cells in different types of cancer such as Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma, Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma with 17p deletion, Waldenström’s Macroglobulinemia, Chronic Graft versus Host Disease.
- In January 2024, the US FDA granted Fast-Track Designation to the novel BTK degrader NX-5948 for the treatment of adult patients with relapsed or refractory chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) after at least two lines of therapy, including a BTK inhibitor and a BCL2 inhibitor.
- Novartis, Ono Pharmaceutical, Beijing Innocare Pharmaceutical, and several other BTK Inhibitors Companies are currently engaged in the development and production of selective BTK inhibitors, which have the potential to significantly impact and enhance the BTKi market.
Request for unlocking the Sample Page of the BTK Inhibitors Market
Equip yourself with key insights by requesting a sample page of our report. Don't wait—click now to learn more @ BTK Inhibitors Market Forecast
DelveInsight’s “BTK Inhibitor Market, Target Population, Competitive Landscape, and Market Forecast – 2034” report delivers an in-depth understanding of the BTK inhibitors, historical and forecasted epidemiology, competitive landscape as well as the BTK inhibitors therapeutics market trends in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
The BTK Inhibitor Market Size report provides current treatment practices, emerging drugs, market share of individual therapies, and current and forecasted 7MM BTK Inhibitor Market Size from 2020 to 2034. The report also covers current BTK inhibitor treatment practices/algorithms and unmet medical needs to curate the best opportunities and assess the market’s potential.
|
Study Period |
2020 to 2034 |
|
Geographies Covered |
|
|
BTK Inhibitors Companies |
|
BTK Inhibitor Market Treatment Market
Bruton’s tyrosine kinase (BTK) inhibitor is a promising novel agent that has potential efficiency in B-cell malignancies. Ever since the structure and function of BTK were clearly defined in 1993, there have been extensive efforts from both industry and academia to develop BTK inhibitors. These inhibitors are primarily being developed as antitumor agents, but their potential applications extend beyond oncology. ibrutinib was the first effective and selective BTK inhibitor approved by the FDA as a breakthrough therapy in 2013. Its approval creates possibilities for an era of chemotherapy-free management of B-cell malignancies, as toxic chemotherapy was the main option for CLL/SLL before its approval.
Overall, BTK inhibitors represent an important class of drugs for the BTK Inhibitor Market Treatment of diseases involving dysregulated B-cell signaling, offering new therapeutic options for patients with hematological malignancies and autoimmune disorders
BTK Inhibitor Market Expression
BTK is highly expressed in B-cell malignancies, such as Chronic Lymphatic Leukemia (CLL), Small Lymphatic Lymphoma (SLL), Mantle Cell Lymphoma (MCL), Waldenstrom’s Macroglobulinemia Lymphoma (WM), Marginal Zone Lymphoma (MZL) and Diffuse Large B-Cell Lymphoma (DLBCL). BTK expression has also been found in various solid tumors, including lung cancer, breast cancer, colon cancer, and oral squamous cell carcinoma. The level of expression may vary depending on the specific tumor type and individual patient. BTK Expression in these solid tumors is not as clear as in B-cell malignancies, while some studies show promise, more research is required to understand the exact role of BTK in each cancer type and determine the effectiveness of BTK inhibitor therapy.
|
Cancer Type |
BTK Expression Category |
|
Chronic Lymphatic Leukemia |
High |
|
Small Lymphatic Lymphoma |
High |
|
Mantle Cell Lymphoma |
High |
|
Waldenstrom’s Macroglobulinemia Lymphoma |
High |
|
Lung Cancer |
Variable |
|
Breast Cancer |
Variable |
|
Colon Cancer |
Variable |
|
Marginal Zone Lymphoma |
Low |
|
Diffuse Large B-cell lymphoma |
Low |
BTK Inhibitor Treatment Landscape
Bruton's tyrosine kinase (BTK) is a protein, specifically an enzyme of the B-cell antigen receptor (BCR) and cytokine receptor pathways. It plays a crucial role in the signaling pathways of B cells. When BCRs on the surface of B cells bind to antigens, they initiate a signaling cascade that ultimately leads to B-cell activation and proliferation. BTK plays a central role in transmitting these signals from the cell surface to the nucleus, where gene expression changes occur.
BTK inhibitors are small molecules designed to bind to the active site of BTK, preventing its enzymatic activity. By blocking BTK, these inhibitors disrupt the downstream signaling pathways that promote B-cell survival, proliferation, and migration leading to inhibition of BTK kinase activity. BTK inhibitors are a type of drug that treat cancers caused by defective B cells, such as chronic lymphocytic leukemia, B-cell lymphomas, and Waldenström macroglobulinemia.
BTK Inhibitor Market Drugs Chapters
The drug chapter segment of the BTK Inhibitor Market Size Reports encloses a detailed analysis of BTK inhibitors marketed drugs and late-stage (Phase III and Phase II) pipeline drugs. It also helps understand the BTK inhibitors' clinical trial details, expressive pharmacological action, agreements and collaborations, approval and patent details, advantages and disadvantages of each included drug and the latest news and press releases.
BTK Inhibitor Marketed Drugs
- JAYPRICA (pirtobrutinib): Eli Lilly/Loxooncol
JAYPRICA (pirtobrutinib) is the first FDA-approved non-covalent Bruton’s tyrosine kinase (BTK) inhibitor for relapsed or refractory mantle cell lymphoma (MCL). It represents a significant advancement in the fight against rare and aggressive blood cancer. It is unique as it is a non-covalent inhibitor, whereas other treatments for MCL on the market are covalent inhibitors and non-covalent inhibitors, It targets specific BTK mutations that are becoming resistant to the covalent inhibitors. This breakthrough medication for MCL is offering new hope to patients who have exhausted other treatment options.
- CALQUENCE (acalabrutinib): Astrazeneca
CALQUENCE (acalabrutinib) is a next-generation, selective inhibitor of Bruton’s tyrosine kinase (BTK). acalabrutinib binds covalently to BTK, thereby inhibiting its activity. In B cells, BTK signaling results in the activation of pathways necessary for B-cell proliferation, trafficking, chemotaxis, and adhesion.
acalabrutinib got its initial approval in 2017 for the capsule dosage form but in 2022, AstraZeneca got approval for the new tablet dosage form. In the trials, results showed the acalabrutinib capsule and tablet formulations are bioequivalent, indicating the same efficacy and safety profile can be expected with the same dosing strength and schedule. The approval of acalabrutinib in a tablet form enables co-administration of the tablet alongside a proton pump inhibitor. This provides another option for some patients with chronic lymphocytic leukemia and relapsed or refractory mantle cell lymphoma, enabling more patients to potentially benefit from this treatment.
|
Product |
Company |
Indication |
|
IMBRUVICA (ibrutinib) |
Pharmacyclics/ Johnson & Johnson |
Treatment of Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma, Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma with 17p deletion, Waldenström’s Macroglobulinemia, Chronic Graft versus Host Disease |
|
CALQUENCE (acalabrutinib) |
Astrazeneca |
Treatment of Mantle Cell Lymphoma, Chronic Lymphocytic Leukemia, or Small Lymphocytic Lymphoma |
|
BRUKINSA (zanubrutinib) |
Beigene |
Treatment of Mantle Cell Lymphoma, Waldenström’s Macroglobulinemia, Marginal Zone Lymphoma, Chronic Lymphocytic Leukemia or Small Lymphocytic Lymphoma, Follicular Lymphoma |
|
JAYPIRCA (pirtobrutinib) |
Loxooncol/Lilly |
Treatment of Mantle Cell Lymphoma, Chronic Lymphocytic Leukemia, or Small Lymphocytic Lymphoma |
BTK Inhibitor Emerging Drugs
- LOU064 (remibrutinib): Novartis
- ONO-4059 (tirabrutinib): Ono pharmaceutical
|
List of Emerging Drugs | |||||
|
Remibrutinib (LOU064) |
Novartis |
Chronic spontaneous urticaria |
BTK inhibitor |
III |
NCT05030311, NCT05030311, NCT05032157 |
|
Novartis |
Multiple Sclerosis |
BTK Inhibitor |
III |
NCT05156281 | |
|
Novartis |
Chronic Inducible Urticaria |
BTK Inhibitor |
II |
NCT05976243 | |
|
Novartis |
Hidradenitis suppurativa |
BTK Inhibitor |
II |
NCT03827798 | |
|
Tirabrutinib (ONO-4059) |
Ono Pharmaceutical |
Primary CNS Lymphoma, Refractory Primary Central Nervous System Lymphoma |
BTK inhibitor |
III |
NCT04947319 |
|
Orelabrutinib (ICP-022) |
Beijing Innocare Pharmatech |
r/rB-cell Malignancies, B-cell Malignancies |
BTK inhibitor |
I/II |
NCT04014205 |
Note: The emerging drug list is indicative, the full list will be given in the final report.
Unveil the crucial information that can shape your strategies. Request a sample report page and get a taste of what's inside @ BTK Inhibitor Market Size
BTK Inhibitor Market Outlook
Over the past decade, numerous preclinical and clinical studies have evaluated the efficacy of BTK inhibitors as single agents or in combination with other standard chemotherapy, immunotherapy, or targeted agents in various cancers to broaden indications and expand markets. Currently approved BTK inhibitors include IMBRUVICA (ibrutinib), CALQUENCE (acalabrutinib), BRUKINSA (zanubrutinib) and JAYPIRCA (pirtobrutinib). ibrutinib was the first effective and selective BTK inhibitor approved by the FDA as a breakthrough therapy in 2013. Subsequently, the second-generation BTK inhibitors acalabrutinib and zanubrutinib, which tried to reduce off-target effects, were approved in 2017 and 2019, respectively.
Honigberg first reported the efficacy of ibrutinib in B-cell lymphoma. Subsequently, to overcome off-target side effects and the emerging resistance of ibrutinib, some selective second-generation BTK inhibitors were developed. Similar to ibrutinib, Harrington evaluated the pharmacodynamic effects of acalabrutinib and demonstrated that acalabrutinib potently inhibited BTK activation, thus inhibiting the proliferation of CLBL1 cells, a canine B-cell lymphoma cell line. The overall response rate (ORR) was 25%, with a median PFS of 22.5 days. zanubrutinib is a next-generation BTK inhibitor developed by BeiGene in 2012. It was designed with the concept of a structure-activity relationship-driven drug design strategy, and compound 31a synthesized in a series of pseudo-pyrimidinone compounds was selected as a potential candidate due to its high potency, selectivity, pharmacokinetics in vitro, and excellent pharmacodynamics.
In 2023, the FDA approved pirtobrutinib which is the first FDA-approved non-covalent Bruton’s tyrosine kinase (BTK) inhibitor for relapsed or refractory mantle cell lymphoma.
A growing body of experimental and clinical data has demonstrated the significance of BTK, not just in B-cell malignancies, but also in solid tumors, such as breast, ovarian, colorectal, and prostate cancers. In addition, enhanced BTK activity is correlated with autoimmune disease. This gave rise to the hypothesis that BTK inhibitors can be beneficial in the therapy of rheumatoid arthritis, systemic lupus erythematosus, multiple sclerosis, Sjögren’s syndromes, allergies, and asthma.
Several key BTK Inhibitors Companies, including Novartis, Ono Pharmaceutical, Beijing Innocare Pharmaceutical, and others, are involved in developing drugs for BTK inhibitors for various indications such as Chronic Spontaneous Urticarial, Multiple Sclerosis, B-cell Malignancies, Primary CNS Lymphoma, Refractory Primary Central Nervous System Lymphoma and others. Overall, this is an exciting new class of agents with great potential for development. The maturation of current studies over the next few years will lead to a better understanding of BTK inhibitors and define their role in the therapy of cancer.
BTK inhibitors Drugs Uptake
This section focuses on the uptake rate of potential approved and emerging BTK inhibitors expected to be launched in the BTK inhibitors market during 2020–2034.
BTK Inhibitors Pipeline Development Activities
The BTK Inhibitors market report provides insights into BTK Inhibitors clinical trials within Phase III, Phase II, and Phase I stage. It also analyzes key BTK Inhibitors Companies involved in developing targeted therapeutics. The presence of numerous BTK Inhibitors drugs under different stages is expected to generate immense opportunity for BTKi market growth over the forecasted period.
BTK Inhibitor Pipeline development activities
The BTK Inhibitor Market Size Report covers information on collaborations, acquisitions and mergers, licensing, and patent details for BTK inhibitors emerging therapies. The increasing strategic collaborations among major market players to enhance the growth of their pipeline products are anticipated to drive market expansion. For example: In July 2021, Biogen and Innocare announced a License and Collaboration Agreement for Orelabrutinib, an Innovative CNS Penetrant BTK Inhibitor for the Potential Treatment of Multiple Sclerosis.
KOL Views on BTK Inhibitors
To keep up with current and future market trends, we take Industry Experts’ opinions working in the domain through primary research to fill the data gaps and validate our secondary research. Industry experts were contacted for insights on BTK inhibitors' evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, drug uptake, along challenges related to accessibility.
DelveInsight’s analysts connected with 25+ KOLs to gather insights; however, interviews were conducted with 10+ KOLs in the 7MM. Centers such as the Division of Hematology at Ohio State University, University Hospitals Seidman Cancer Center, Case Comprehensive Cancer Center in Cleveland, and others were contacted.
Their opinion helps understand and validate current and emerging therapy treatment patterns or BTK inhibitors market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs for the BTK Inhibitor Market.
|
KOL Views |
|
“JAYPRICA (pirtobrutinib) is now available, and (the noncovalent BTK inhibitor) represents a new class of drugs. It does inhibit the same target as some of the previously approved (BTK inhibitors), but it hits BTK differently; therefore, patients have an additional option (after a prior covalent BTK inhibitor). In addition to being quite effective in patients whose disease relapsed on a previous covalent BTK inhibitor, pirtobrutinib is quite remarkable for having a very favorable safety profile. For example, it doesn't have the cardiac toxicity associated with previously approved BTK inhibitors.” |
- Many Pharma BTK Inhibitors Companies presented the data of their BTK inhibitors during the ASCO 2023 conference including Ono Pharmaceuticals and others. Ono’s study concluded that Tirabrutinib showed long-lasting effectiveness in a subset of patients with a tolerable safety profile of rrPCNSL
- In January 2024, the US FDA granted Fast-Track Designation to the novel BTK degrader NX-5948 for the treatment of adult patients with relapsed or refractory chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) after at least two lines of therapy, including a BTK inhibitor and a BCL2 inhibitor
- Scope of the Report
- The BTK Inhibitors market report covers a segment of key events, an executive summary, and a descriptive overview of BTK inhibitors, explaining its mechanism, and therapies (current and emerging).
- Comprehensive insight into the Competitive Landscape, and forecasts, the future growth potential of treatment rate, drug uptake, and drug information have been provided.
- Additionally, an all-inclusive account of the current and emerging therapies and the elaborative profiles of late-stage and prominent therapies will impact the current landscape.
- A detailed review of the BTK inhibitors market, historical and forecasted BTK Inhibitors market size, BTK Inhibitors market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The BTK Inhibitors market size report provides an edge while developing business strategies, by understanding trends, through SWOT analysis expert insights/KOL views, and treatment preferences that help shape and drive the 7MM BTK inhibitors market
BTK Inhibitors Market Forecast Report Insights
- BTK Targeted Patient Pool
- BTK Inhibitors Therapeutic Approaches
- BTK inhibitors Pipeline Analysis
- BTK Inhibitors Market Size and Trends
- Existing and future Market Opportunity
BTK Inhibitors Market Report Key Strengths
- 11 years BTK Inhibitors Market Forecast
- The 7MM Coverage
- Key Cross Competition
- BTK Inhibitors Drugs Uptake
- Key BTK Inhibitors Market Forecast Assumptions
BTK Inhibitors Treatment Market Report Assessment
- Current BTK Inhibitors Treatment Market Practices
- BTK Inhibitor Unmet Needs
- BTK Inhibitors Pipeline Product Profiles
- BTK Inhibitors Market Attractiveness
- Qualitative Analysis (SWOT)
- BTK Inhibitors Market Drivers
- BTK Inhibitors Market Barriers
Key Questions Answered In The BTK Inhibitors Market Report
- What was the BTK inhibitor market size, the BTK Inhibitors market size by therapies, BTK Inhibitors market share (%) distribution, and what would it look like in 2034? What are the contributing factors for this growth?
- Which BTK Inhibitor drug is going to be the largest contributor in 2034?
- Which is the most lucrative market for BTK inhibitors?
- Which drug type segment accounts for maximum BTK inhibitor sales?
- What are the pricing variations among different geographies for approved therapies?
- How has the reimbursement landscape for BTK inhibitors evolved since the first one was approved? Do patients have any access issues that are driven by reimbursement decisions?
- What are the risks, burdens, and unmet needs of treatment with BTK inhibitors? What will be the growth opportunities across the 7MM for the patient population of BTK inhibitors?
- What are the key factors hampering the growth of the BTK inhibitors market?
- What are the indications for which recent novel therapies and technologies have been developed to overcome the limitations of existing treatments?
- What key designations have been granted to the therapies for BTK inhibitors?
- What is the cost burden of approved BTK Inhibitor therapies on the patient?
- Patient acceptability in terms of preferred therapy options as per real-world scenarios?
- What are the country-specific accessibility issues of expensive, recently approved BTK Inhibitor therapies?
Reasons to Buy BTK Inhibitors Market Forecast Report
- The BTK Inhibitors market size report will help develop business strategies by understanding the latest trends and changing dynamics driving the BTK inhibitors Market.
- Understand the existing BTK Inhibitors market opportunities in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain) the United Kingdom, and Japan.
- Identifying strong upcoming BTK Inhibitors Companies in the BTK Inhibitors market will help devise strategies to help get ahead of competitors.
- Detailed analysis and ranking of indication-wise current and emerging therapies under the conjoint analysis section to provide visibility around leading indications.
- Highlights of Access and Reimbursement policies of approved therapies, barriers to accessibility of expensive off-label therapies, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing BTK Inhibitors market so that the upcoming BTK Inhibitor companies can strengthen their development and launch strategy.
Click here to view the latest blogs:
- Eli’s Jaypirca: A Beacon of Success in the BTK Inhibitor Market
- Pfizer succeeds COVID-19 vaccine safety milestone; Sanofi snags speedy review for BTK drug; Autoimmune diseases research updates; Elevation Oncology secures $65 Million
- Bruton's Tyrosine Kinase (BTK) Inhibitors An Emerging Therapeutic Target
- Latest Delveinsight Blogs