
A brief preview of upcoming NSCLC updates at ESMO 2024.
- Home
- esmo conference 2024
- nsclc at esmo 2024
NSCLC at ESMO 2024
A quick look at what's coming for NSCLC at ESMO 2024?
Several pharmaceutical companies are set to showcase potential practice-changing research and next-generation candidates in non-small cell lung cancer (NSCLC) at the European Society for Medical Oncology (ESMO) Congress 2024, which will be held September 13-17 in Barcelona. Data from more than 15 company-sponsored, investigator-sponsored and collaborative research abstracts, including oral, mini-oral presentations, proffered paper session, and poster, will be presented at the ESMO Congress 2024. Significant developments are anticipated from this year's congress for NSCLC, ranging from early to advanced stage NSCLC. ESMO 2024 congress will provide practice-changing results in NSCLC, ranging from immunotherapies, novel tyrosine kinase inhibitors (TKIs), antibody-drug conjugates (ADCs), novel therapies and first-in-human trial data.
NSCLC has always been a key indication for oncology key players, because it is the most prevalent form of lung cancer, accounting for approximately 85% of all lung cancer cases. DelveInsight estimates that the total incident population of NSCLC in the United States is around 200,000 (2023 estimates). NSCLC can be categorized into three primary subtypes: adenocarcinoma, squamous cell carcinoma, and large cell carcinoma. Adenocarcinoma, the most common subtype, occurs in about 40% of cases and is typically found in non-smokers. Squamous cell carcinoma and large cell carcinoma occur in approximately 25% and 10% of NSCLC patients, respectively. NSCLC is often diagnosed at advanced stages, with roughly 60% of patients presenting with metastatic or advanced disease. In the United States, there are about 110,000 (2023 estimates) metastatic or advanced NSCLC cases.
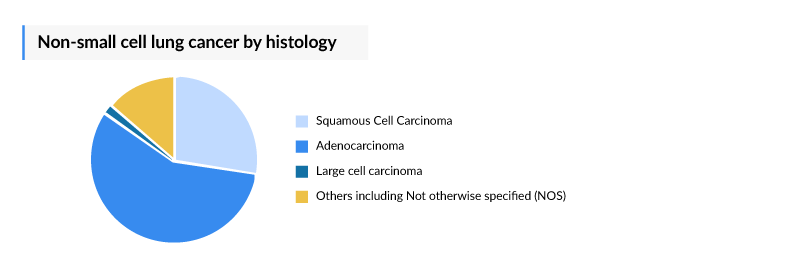
Approximately 50% of NSCLC cases are driven by known genetic mutations, with KRAS, EGFR, and ALK being the most common. Historically, treatment options for NSCLC were limited to surgery, chemotherapy, and radiotherapy. However, recent advances in precision medicine, targeted therapies, and immunotherapy have revolutionized NSCLC management. Treatments such as EGFR inhibitors, ALK-targeted therapies, and PD-L1 inhibitors have significantly improved patient outcomes, especially for those with specific oncogenic drivers. The prognosis for NSCLC patients varies widely depending on the diagnosis stage and targeted treatments' availability. Early-stage localized NSCLC offers a 5-year survival rate of around 60%, while metastatic NSCLC has a dismal 5-year survival rate of only 6%. However, as personalized treatment options evolve, overall survival continues to improve, particularly for patients with actionable mutations.
At ESMO 2024, key developments include Bristol Myers Squibb and Mirati Therapeutics advancing KRAS inhibitors, with Pfizer and AbbVie focusing on BRAF kinase inhibitors and ADCs. Companies like Nuvalent, Johnson & Johnson, and Dizal Pharmaceutical are also set to present data for their ROS1 inhibitor, ALK inhibitor, bispecific antibodies, and EGFR inhibitor, respectively. In addition to this, Cantargia will present data for their IL1RAP inhibitor (nadunolimab), while emerging data on PD-1 inhibitor (Dostarlimab) in combination with TIGIT inhibitor (Belrestotug) from iTeos promises to enrich the therapeutic options for NSCLC. This array of development reflects a concerted push towards targeted and combination therapies aimed at improving outcomes for patients with early and advanced NSCLC.
- Abstract Number – LBA57
- Abstract Type – Mini Oral session
- Indication - NSCLC
Title: Insights from ESMO 2024 on KRAZATI for KRAS G12C-Mutated Patients
Executive Summary: ESMO 2024 will spotlight Phase III KRYSTAL-12 trial results for KRAZATI (adagrasib) in KRAS G12C-mutated NSCLC, comparing its efficacy against docetaxel, particularly in patients with brain metastases, offering potential advancements in treatment for this challenging population.
Main Content –
Among the key drivers of NSCLC progression is the KRAS G12C mutation, a biomarker associated with poor prognosis, found in around 14% of patients with lung adenocarcinoma. The emergence of targeted therapies like KRAZATI has transformed the treatment landscape for patients harboring this mutation.
KRAZATI is approved for use in adult patients with KRAS G12C-mutated cancers based on specific indications and prior treatment history. As a monotherapy, KRAZATI is indicated for the treatment of KRAS G12C-mutated locally advanced or metastatic NSCLC in patients who have undergone at least one prior systemic therapy.
At ESMO 2024, Bristol-Myers Squibb’s much-anticipated Phase III KRYSTAL-12 trial data on KRAZATI will be a key highlight. The study compares KRAZATI to docetaxel in patients with KRAS G12C-mutated, locally advanced or metastatic NSCLC who have undergone prior systemic therapy. The trial particularly focuses on patients with baseline brain metastases, a subgroup with poor outcomes and limited treatment options.
The results from this trial will offer critical insights into whether KRAZATI can outperform docetaxel, which is typically associated with suboptimal responses and significant side effects in this patient group. The inclusion of patients with brain metastases could also shine a light on KRAZATI’s central nervous system (CNS) activity, potentially positioning it as a game-changing option for patients with advanced disease and limited therapeutic alternatives.
- Abstract Number – 1302P
- Abstract Type – Poster
- Indication - NSCLC
Title: Promising Early Data on Regeneron’s Davutamig (MET x MET) for MET-Altered Advanced NSCLC
Executive Summary: At ESMO 2024, preliminary results from a first-in-human study of davutamig (REGN5093), a bispecific antibody targeting MET alterations in advanced NSCLC, show a favorable safety profile and promising efficacy. This novel therapy could offer new treatment options for patients with limited alternatives.
Main Content –
At ESMO 2024, a notable update on davutamig, a novel human bispecific antibody targeting MET alterations in advanced NSCLC, will be presented. This first-in-human study explores the safety, tolerability, and antitumor activity of davutamig in patients with unresectable or metastatic NSCLC lacking standard treatment options. There is an unmet need for patients with MET-altered advanced NSCLC.
Davutamig uniquely binds to two distinct epitopes of the MET receptor, facilitating rapid internalization and degradation of the receptor. By blocking ligand binding, it effectively disrupts the MET signaling pathway that promotes tumor cell proliferation.
The study design features a dose-escalation phase, where patients received davutamig at doses of 500, 1000, and 2000 mg via intravenous infusion every three weeks. Following this, a cohort expansion phase at the 2000 mg dose was initiated, targeting patients with confirmed MET alterations, including exon 14 skipping mutations, gene amplification, and protein overexpression. Tumor assessments were conducted every six weeks until disease progression or study completion.
Early data indicate that davutamig exhibits a favorable safety profile, alongside promising preliminary efficacy signals in patients with MET-altered advanced NSCLC. This innovative approach holds potential for significantly improving outcomes in a patient population with limited treatment alternatives.
As the landscape of NSCLC continues to evolve, the insights from this study may pave the way for new therapeutic strategies targeting MET alterations, highlighting the importance of personalized medicine in oncology.
- Abstract Number – 1261P
- Abstract Type – Poster
- Indication - NSCLC
Title: Evaluating Glecirasib and JAB-3312 Combination for KRAS G12C Mutant NSCLC
Executive Summary: ESMO 2024 will unveil new data on the combination of glecirasib and JAB-3312 for front-line treatment of KRAS G12C mutant NSCLC, demonstrating promising efficacy and safety. The findings will further explore the impact of PD-L1 expression and co-mutations on therapeutic outcomes.
Main Content –
At ESMO 2024, new insights will be presented by Jacobio Pharma regarding the combination of glecirasib (a KRAS G12C inhibitor) and JAB-3312 (an SHP2 inhibitor) as a front-line treatment for NSCLC patients harboring KRAS G12C mutations. Following promising findings at ESMO 2023 and ASCO 2024, this combination has shown a favorable safety profile and encouraging efficacy.
The ongoing Phase I/IIa study (NCT05288205) investigates various dosing regimens of glecirasib and JAB-3312 in patients with KRAS p.G12C mutated solid tumors. Key efficacy endpoints include the ORR and PFS. Importantly, tumor cell proportion score (TPS) data for PD-L1 expression was gathered from both local laboratories and central lab analyses of baseline tumor samples, allowing for a comprehensive assessment of response relative to PD-L1 levels.
Initial findings indicate that the combination of glecirasib and JAB-3312 achieves a favorable ORR in KRAS p.G12C mutated NSCLC, independent of PD-L1 expression levels. Notably, co-mutations involving the SMARC family may be linked to poorer prognosis in this patient population, underscoring the need for further exploration of biomarker-driven outcomes.
More comprehensive data will be unveiled at ESMO 2024, providing crucial insights into the potential of this novel therapeutic combination in managing KRAS G12C mutated NSCLC. This will be pivotal for understanding the implications of PD-L1 expression and co-mutations on treatment efficacy, offering hope for tailored strategies in this challenging patient cohort.
Some other notable abstracts for NSCLC
Executive Summary
Several Pharmaceutical companies are set to showcase potential practice-changing research and next-generation candidates in Non-small cell lung cancer (NSCLC) at the European Society for Medical Oncology (ESMO) Congress 2024, being held September 13-17 in Barcelona.