CAR T-Cell Therapy for Non-Hodgkin's lymphoma Market
- CAR T-Cell therapy has transformed the treatment of aggressive lymphomas such as DLBCL and is now a viable option for RR-FL; however, follow-up remains limited.
- Identifying appropriate patients for CAR T-cell therapy stands out as a paramount challenge, given its expensive nature and aggressive treatment approach.
- The FDA has approved four CAR-Ts in different subtypes of NHL, such as YESCARTA (DLBCL and follicular lymphoma), KYMRIAH (DLBCL and follicular lymphoma), BREYANZI (DLBCL), and TECARTUS (MCL).
- In March 2024, Bristol Myers Squibb announced that the US FDA approved BREYANZI as the first and only CAR T Cell Therapy for adults with R/R CLL or SLL.
- In May 2024, Verismo Therapeutics received IND clearance from the FDA for SynKIR-310 in R/R B-cell NHL.
- YESCARTA is the only CAR-T being evaluated in 1L DLBCL.
- KYMRIAH's revenue is anticipated to face challenges in achieving steady growth within the DLBCL market across the 7MM, largely due to stiff competition posed by Gilead's YESCARTA and BMS's BREYANZI. However, its revenue from emerging markets is projected to experience growth.
- TECARTUS is the only CD-19 CAR-T approved in 3L MCL.
- BREYANZI is poised to dominate the CAR-T Market in NHL in the forthcoming years, with potential approvals anticipated across three indications: CLL/SLL, follicular lymphoma, and MCL. Notably, it has already secured approval for DLBCL in 3L+, 2L+ TE, and NTE.
- Few treatment alternatives for patients who relapse after CAR-T are available.
- In the emerging pipeline, most of the companies are focusing on B-cell NHL only a handful of players targeting T-cell NHL, such as Autolus Therapeutics, CRISPR Therapeutics, iCell Gene Therapeutics, Legend Biotech, and others.
- Novartis is progressing with its additional CD-19 CAR-T therapy, Rapcabtagene autoleucel, with expectations of a significantly reduced turnaround time for availability compared to KYMRIAH.
- CAR T-Cell Therapy for Non-Hodgkin's lymphoma Companies are leveraging the strength of dual and triple targeting in CAR-T therapies. Miltenyi Biomedicine, Gilead Sciences, 2seventy bio, and Novartis are developing CD19/20 Bispecific CAR-T, CD79a/CD20 Bispecific CAR-T, and CD2xCD3xCD19 CAR-T, enhancing treatment efficacy by targeting multiple antigens simultaneously.
- Approximately 90% of the overall CAR-T Market is projected to be dominated by Aggressive B-cell NHL, with the remaining 10% attributed to indolent B-cell NHL.
Request for unlocking the sample page of the "CAR T-Cell Therapy for Non-Hodgkin's lymphoma Treatment Market"
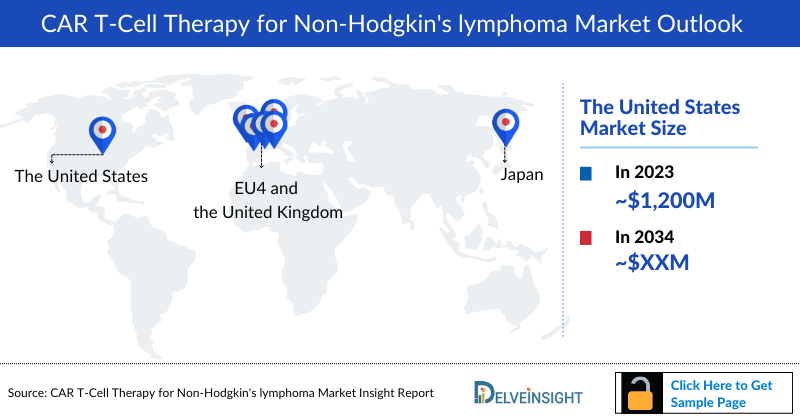
DelveInsight’s “CAR T-Cell Therapy for Non-Hodgkin's lymphoma Market Insights, Epidemiology and Market Forecast – 2034” report delivers an in-depth understanding of the CAR-T in NHL, historical and forecasted epidemiology as well as the CAR-T in NHL market trends in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
The CAR T-Cell Therapy for Non-Hodgkin's lymphoma Treatment Market report provides current treatment practices, emerging drugs, market share of individual therapies, and current and forecasted 7MM CAR T-Cell Therapy for Non-Hodgkin's lymphoma market size from 2020 to 2034. The report also covers current CAR T-Cell Therapy for Non-Hodgkin's lymphoma treatment market practices/algorithms and unmet medical needs to curate the best opportunities and assess the market’s underlying potential.
|
Study Period |
2020 to 2034 |
|
Forecast Period |
2024-2034 |
|
Geographies Covered |
|
|
CAR T-Cell Therapy for Non-Hodgkin's lymphoma Market |
|
|
CAR T-Cell Therapy for Non-Hodgkin's lymphoma Market Size | |
|
CAR T-Cell Therapy for Non-Hodgkin's lymphoma Companies |
|
Key Factors Driving the CAR T-Cell Therapy for Non-Hodgkin's Lymphoma Market
Rise in NHL Incidence
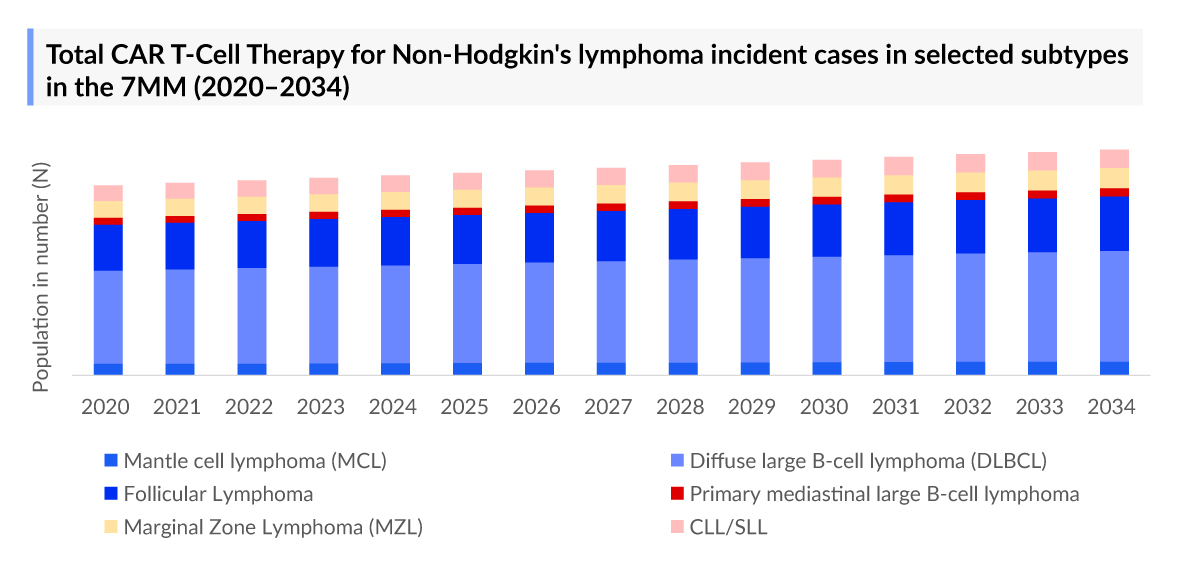
The global incidence of non-Hodgkin’s lymphoma is rising, driven by aging populations, lifestyle changes, and environmental risk factors. In the year 2023, the total NHL incident cases in selected subtypes were ~155K cases in the 7MM.
Dominance of Gilead Sciences
Gilead Sciences is the major player in the CAR-Ts as already two CAR-Ts are approved, i.e., YESCARTA and TECARTUS, and two in the early stage of development (KITE-363 and KITE-363).
CAR T-Cell Therapy for Non-Hodgkin's Lymphoma Competitive Landscape
Some of the drugs in the pipeline include Cemacabtagene ansegedleucel (Allogene Therapeutics), Rapcabtagene autoleucel (Novartis), Zamtocabtagene autoleucel (Miltenyi Biomedicine), MB-106 (Mustang Bio), Azercabtagene zapreleucel (Imugene), and others.
Emergence of Novel Targets
The exploration of novel targets beyond CD19 in the CAR-T pipeline offers significant opportunities. Companies like Autolus Limited, PeproMene Bio, and CRISPR Therapeutics are pioneering research into targets such as TRBC1, BAFF-R signaling, and CD70, potentially broadening treatment options for patients.
CAR T-Cell Therapy for Non-Hodgkin's lymphoma Treatment Market
CAR-T cell therapy stands as a revolutionary approach in cancer immunotherapy, particularly for certain NHL that have proven resistant to conventional treatments. By harnessing the power of genetically modified T cells, this cutting-edge therapy enhances the immune system's ability to target and destroy cancer cells. Specifically, CAR-T cell therapy involves the genetic modification of a patient's T cells to express chimeric antigen receptors (CARs) on their surface. These engineered CARs are designed to recognize and bind to specific proteins present on the surface of cancer cells, effectively directing the immune response toward eradicating the malignancy.
The treatment landscape for NHL is multifaceted and dependent on various factors including the subtype of NHL, disease stage, overall health, and patient preferences. CAR-T cell therapy has emerged as a promising avenue within this landscape, particularly for aggressive B-cell NHLs that have proven refractory to standard therapies. Historically, such cases have presented significant challenges, with poor prognoses despite salvage chemotherapy and autologous stem cell transplant. However, the advent of CAR-T cell therapy has transformed this outlook, offering remarkable response rates and the potential for durable remissions even in patients with advanced disease progression following multiple prior treatments. The FDA's approval of several CAR-T therapies for NHL underscores the significance of this breakthrough. YESCARTA, KYMRIAH, BREYANZI, and TECARTUS have each demonstrated efficacy across different subtypes of NHL, including DLBCL, follicular lymphoma, and mantle cell lymphoma (MCL). Ongoing research endeavors aim to refine CAR-T cell therapy for NHL further, striving to enhance efficacy, minimize toxicities, and broaden the spectrum of target antigens beyond CD19. Moreover, investigations exploring the synergistic potential of combining CAR-T cell therapy with other modalities such as checkpoint inhibitors or targeted agents are underway, with the goal of maximizing therapeutic outcomes for patients battling NHL.
CAR T-Cell Therapy for Non-Hodgkin's lymphoma Epidemiology
As the market is derived using a patient-based model, the CAR-T in NHL epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by Total Incident Cases of Selected Indications for CAR-T in NHL (Mantle cell lymphoma, DLBCL, Follicular lymphoma, Marginal Zone Lymphoma, Primary mediastinal large B-cell lymphoma, Chronic lymphocytic leukemia/small-cell lymphocytic lymphoma) and Total Indication wise Eligible Cases of CAR-T in NHL, in the 7MM covering the United States, EU4 countries (Germany, France, Italy, and Spain), United Kingdom, and Japan from 2020 to 2034.
- As per DelveInsight's estimates, in 2023, the total eligible cases of NHL for CAR-T were around 125,900 in the 7MM.
- DLBCL stands out as the most prevalent subtype among selected types of non-Hodgkin lymphoma in EU4 and UK, with approximately 32,400 cases reported in 2023.
- In 2023, Japan recorded approximately 23,700 cases of total NHL incident cases in selected subtypes in 2023.
CAR-T in NHL Drug Chapters
The drug chapter segment of the CAR-T in NHL encloses a detailed analysis of CAR-T in NHL-marketed drugs and late-stage (Phase III and Phase II) CAR T-Cell Therapy for Non-Hodgkin's lymphoma pipeline drugs analysis. It also helps understand the CAR T-Cell Therapy for Non-Hodgkin's lymphoma clinical trials details, expressive pharmacological action, agreements and collaborations, approval and patent details, advantages and disadvantages of each included drug, and the latest CAR T-Cell Therapy for Non-Hodgkin's lymphoma news and press releases.
CAR T-Cell Therapy for Non-Hodgkin's lymphoma Marketed Drugs
- YESCARTA (axicabtagene ciloleucel): Gilead Sciences (Kite Pharma)
YESCARTA is a CD19-directed genetically modified autologous T-cell immunotherapy. Patient’s T cells are harvested and genetically modified ex vivo by retroviral transduction to express a chimeric antigen receptor (CAR) comprising a murine anti-CD19 single-chain variable fragment (scFv) linked to CD28 and CD3-zeta costimulatory domains. The anti-CD19 CAR-T cells are expanded and infused back into the patient, where they can recognize and eliminate CD19-expressing target cells. YESCARTA binds to CD19-expressing cancer cells and normal B cells.
Moreover, in June 2022, YESCARTA was approved for adult patients with relapsed or refractory follicular lymphoma after three or more lines of systemic therapy.
- TECARTUS (brexucabtagene autoleucel): Gilead Sciences (Kite Pharma)
TECARTUS is a CD19-directed genetically modified autologous T cell immunotherapy, which binds to CD19-expressing cancer cells and normal B cells. Studies demonstrated that following anti-CD19 CAR T cell engagement with CD19-expressing target cells, the CD28 and CD3-zeta costimulatory domains activate downstream signaling cascades that lead to T cell activation, proliferation, acquisition of effector functions, and secretion of inflammatory cytokines and chemokines. This sequence of events leads to the killing of CD19-expressing cells. It is indicated for the treatment of adult patients with R/R MCL.
|
Comparison of Marketed Drugs | |||||
|
CAR-T |
Company Name |
MoA |
RoA |
Molecule Type |
Approval Year |
|
YESCARTA |
Gilead Sciences (Kite Pharma) |
CD19-directed genetically modified autologous T-cell immunotherapy |
IV |
Autologous CAR-T-cell therapy |
US: (2017) (2021) (2022); EU: (2018) (2022); JP: (2021) |
|
KYMRIAH |
Novartis |
CD19-targeted CAR T-cell immunotherapy |
IV |
Autologous CAR-T-cell therapy |
US (2018) (2022); EU (2018) (2022); JP: (2019) (2022) |
|
BREYANZI |
BMS |
CD19-directed genetically modified autologous T-cell immunotherapy |
IV |
Autologous CAR-T-cell therapy |
US: (2021) (2022) (2024); EU: (2022); JP (2021) |
|
TECARTUS |
Gilead Sciences (Kite Pharma) |
CD19-directed genetically modified autologous T-cell immunotherapy |
IV |
Autologous CAR-T-cell therapy |
US: (2020); EU: (2020) |
Note: Detailed current therapies assessment will be provided in the full report of CAR-T in the NHL...
CAR T-Cell Therapy for Non-Hodgkin's lymphoma Emerging Drugs
- Cemacabtagene ansegedleucel: Allogene Therapeutics
Cemacabtagene ansegedleucel (ALLO-501A), a next-generation anti-CD19 AlloCAR-T, is engineered without the rituximab recognition domains included in ALLO-501, which could allow for use in a broader patient population, including NHL patients with recent rituximab exposure; cemacabtagene ansegedleucel uses the Cellectis TALEN technology. The company is conducting long-term follow-up in the Phase I clinical trial (the ALPHA trial) of ALLO-501 in patients with R/R NHL. The company has also initiated a Phase I/II pivotal clinical trial for ALLO-501A (ALPHA2 trial) in the second quarter of 2020.
In June 2022, Allogene announced that the US FDA granted RMAT designation to cemacabtagene ansegedleucel in R/R LBCL. In 2021, the US FDA granted FTD status to cemacabtagene ansegedleucel for treating adult patients with R/R DLBCL.
The company’s recent presentation highlights several key developments in its clinical trials. The initial data readout for the Phase I ALPHA2 trial in relapsed/refractory chronic lymphocytic leukemia (R/R CLL) is anticipated in 2024, with patient enrollment currently ongoing. Additionally, the pivotal Phase II ALPHA3 trial for first-line consolidation treatment of LBCL was launched in June 2024. This trial is expected to complete enrollment in the first half of 2026, with a potential BLA submission planned for 2027. In July 2024, the company activated three community cancer centers as the first sites for the pivotal Phase II ALPHA3 trial, which is evaluating Cemacabtagene Ansegedleucel as a first-line consolidation treatment for LBCL patients.
- Rapcabtagene autoleucel (YTB323): Novartis
Rapcabtagene autoleucel, an investigational, autologous CD19-directed CAR-T-cell therapy developed using the T-charge platform, showed promising results in the DLBCL arm of a first-in-human, multicenter, Phase I dose-escalation study. The company is currently evaluating YTB323 in a Phase II trial for patients with first-line high-risk large B-cell lymphoma. They anticipate filing for approval by 2027 or later.
Note: Detailed emerging therapies assessment will be provided in the final report.
|
Comparison of Emerging Drugs Under Development | |||||
|
CAR-T |
Company |
Phase |
Indication |
MoA |
Molecule Type |
|
Cemacabtagene ansegedleucel |
Allogene Therapeutics |
II |
R/R LBCL |
Targeting CD19 |
Allogeneic CAR-T cell therapy |
|
Rapcabtagene autoleucel |
Novartis |
II |
1L high-risk large B-cell lymphoma |
Targeting CD19 |
Autologous CAR-T cell therapy |
|
Zamtocabtagene autoleucel |
Miltenyi Biomedicine |
II |
R/R DLBCL |
Targeting the combination of CD19 and CD20 |
Autologous CAR-T cell therapy |
|
AUTO4 |
Autolus Therapeutics |
I/II |
R/R T-NHL, PTCL, AITL, ALCL |
Targeting TRBC1 |
Autologous CAR-T cell therapy |
|
CD30.CAR-T |
Tessa Therapeutics |
I |
R/R ALCL, PTCL, Extranodal NK/T-cell Lymphoma, DLBCL, PMBCL |
Targeting CD30 |
Allogeneic CAR-T cell therapy |
|
PMB-CT01 |
PeproMene Bio |
I |
R/R B-cell ALL |
Targeting BAFF-R signaling |
Autologous CAR-T cell therapy |
Note: Detailed list will be provided in the final report.CAR-T in NHL Market Outlook
The CAR T-Cell Therapy for Non-Hodgkin's lymphoma emerging pipeline includes late, mid, and early-stage drugs in different lines of therapies and different indications, mainly including B- cell Lymphoma such as DLBCL, FL, MCL, MZL, CLL/SLL, and others, with one of them being developed for a T-cell Lymphoma, PTCL. Considering the scope of CAR T-cell investigations in NHL As of now, there are four CAR-Ts approved in NHL: YESCARTA, KYMRIAH, BREYANZI, and TECARTUS.
The majority of advancements in the NHL sector focus on DLBCL within the CAR-T Pipeline. While YESCARTA has obtained approval in several DLBCL segments, it is not approved for 1L treatment and 2L+ NTE patients in the United States. Consequently, the company is conducting trials in both of these segments. In the emerging DLBCL pipeline, various CAR-T therapies such as zamtocabtagene autoleucel, ALLO-501A, YTB323, and others are in different phases of trials and cater to different patient segments.
In the domain of indolent NHL, another subtype known as MZL is gaining attention. Both YESCARTA and BREYANZI are currently undergoing trials in MZL, mirroring the trials conducted in follicular lymphoma. YESCARTA has already obtained approval for follicular lymphoma, and BREYANZI is anticipated to receive approval in the coming years based on similar trials. Currently no available data for BREYANZI and YESCARTA in this indication. Upon approval of both CAR-T therapies in MZL, the treatment landscape for this condition is expected to undergo significant transformation.
Currently, autologous CAR-T therapies are the only ones approved, but the emerging pipeline predominantly comprises autologous CAR-Ts alongside a growing number of allogeneic CAR-T therapies. Several companies advancing allogeneic CAR-T cell therapies include Allogene Therapeutics (ALLO-501A), Wugen (WU-CART-007), Imugene (PBCAR0191), and others.
- YESCARTA was the first CAR-T approved for the treatment of follicular lymphoma. Later, in 2022, KYMRIAH was approved for the same.
- The total CAR T-Cell Therapy for Non-Hodgkin's lymphoma Treatment Market Size in the United States was around USD 1,200 million in 2023, expected to rise by 2034.
- Among the EU4, Germany captured the maximum CAR T-Cell Therapy for Non-Hodgkin's lymphoma Drugs Market Share in 2023, whereas Spain was at the bottom of the ladder in the same year.
- The FDA has approved four CAR-Ts in different subtypes of NHL, such as YESCARTA (DLBCL and follicular lymphoma), KYMRIAH (DLBCL and follicular lymphoma), BREYANZI (DLBCL), and TECARTUS (MCL).
- YESCARTA appears to have the upper hand in the battle for CAR-T supremacy, given it has been in the market before BREYANZI. Currently, YESCARTA captures the highest patient share among all CAR-T therapies.
CAR T-Cell Therapy for Non-Hodgkin's lymphoma Drugs Uptake
This section focuses on the uptake rate of potential CAR T-Cell Therapy for Non-Hodgkin's lymphoma therapies expected to be launched in the market during 2024–2034, which depends on the competitive landscape, safety, and efficacy data along with order of entry. It is important to understand that the key players evaluating their novel therapies in the pivotal and confirmatory trials should remain vigilant when selecting appropriate comparators to stand the greatest chance of a positive opinion from regulatory bodies, leading to approval, smooth launch, and rapid uptake. Among the selected therapies, YESCARTA is expected to generate the maximum revenue by 2034 in the 7MM
Further detailed analysis of emerging therapies uptake in the report
CAR-T in NHL Pipeline Development Activities
The CAR T-Cell Therapy for Non-Hodgkin's lymphoma therapeutics market report provides insights into different therapeutic candidates in Phase III and Phase II stages. It also analyzes key CAR T-Cell Therapy for Non-Hodgkin's lymphoma Companies involved in developing targeted therapeutics.
Pipeline Development Activities
The report covers information on collaborations, acquisitions and mergers, licensing, and patent details for CAR-T in NHL emerging therapies. The landscape of pipeline activity in DLBCL appears robust, characterized by the involvement of numerous entities such as Allogene Therapeutics, Miltenyi Biomedicine, CRISPR Therapeutics, 2seventy bio, and others, who are actively assessing their CAR-T therapies in the third-line setting. Concurrently, established CAR-T therapies are advancing into earlier lines of treatments.
Take Your Research to the Next Level! Click Here to Get Access to the Full Pipeline Report @ CAR T-Cell Therapy for Non-Hodgkin's lymphoma Treatment Drugs
KOL Views
To keep up with current market trends, we take KOLs and SMEs’ opinions working in the domain through primary research to fill the data gaps and validate our secondary research. Industry Experts contacted for insights on CAR-T in NHL's evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and uptake of therapy, along with challenges related to accessibility, including oncologists, radiation oncologists, surgical oncologists, and others.
Delveinsight’s analysts connected with 30+ KOLs to gather insights; however, interviews were conducted with 15+ KOLs in the 7MM. Centers such as MD Anderson Cancer Center, MD Center for Lymphoma, Division of Hematology & Oncology, Department of Hematology, National Cancer Center, etc., were contacted. Their opinion helps understand and validate current and emerging therapy treatment patterns for CAR-T in NHL market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
|
KOL Views |
|
“Indolent non-Hodgkin lymphoma is a slow-developing chronic disease in which patients frequently relapse, which leads to the need for new treatment strategies. It is encouraging to see that axi-cel provided a continued benefit over two years and may provide a lasting treatment for these patients.” –MD at Anderson Cancer Center, the US |
|
“B-cell non-Hodgkin lymphoma (NHL) is the most frequent hematologic malignancy. Despite the refinement of chemoimmunotherapy, a substantial number of patients experience chemorefractory disease. Anti-CD19 CAR T-cell therapy is considered the most promising and effective therapy to overcome chemorefractory B-cell NHL.” –Department of Hematology, National Cancer Center Hospital, Japan |
|
“Most salvage chemotherapy regimens will have some effect in upwards of half of people with aggressive B-cell NHL, but salvage chemotherapy alone usually does not lead to long-lasting remission.” –MD of NCI’s Center for Cancer Research, the US |
CAR T-Cell Therapy for Non-Hodgkin's lymphoma Drugs Market: Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT analysis and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of gaps in disease diagnosis, patient awareness, physician acceptability, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided.
Conjoint Analysis analyzes multiple approved and emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy. In efficacy, the trial’s primary and secondary outcome measures are evaluated; for instance, in event-free survival, one of the most important primary outcome measures is event-free survival and overall survival.
Further, the therapies’ safety is evaluated wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
CAR T-Cell Therapy for Non-Hodgkin's lymphoma Therapeutics Market Access and Reimbursement
In February 2018, the ICER assessed the comparative clinical effectiveness and value of tisagenlecleucel and axicabtagene ciloleucel. Both therapies improved response rates and survival for patients who have exhausted most other treatment options; the drugs were priced in alignment with their clinical value. California Technology Assessment Forum (CTAF) is one of ICER’s three independent evidence appraisal committees that quoted that the evidence is limited for each drug, as its respective indication provided small to substantial net health benefits compared to commonly-used chemotherapies. For KYMRIAH, with a list price of USD 475,000, the Medicare payment rate as of April 1 is USD 500,839.
Kite Konnect provides support for eligible patients receiving YESCARTA and TECARTUS, and it provides information for the healthcare teams supporting their patients. In Spain, KYMRIAH got surprisingly quick approval for reimbursement from the Spanish healthcare system in December 2018 and was reimbursed in the Spanish NHS through two outcomes-based, staged payments based on data collected through the Valtermed system.
The CAR T-Cell Therapy for Non-Hodgkin's lymphoma therapeutics market report provides detailed insights on the country-wise accessibility and reimbursement scenarios, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
ASCO 2024 Highlights
Autolus Therapeutics presented long-term follow-up and additional data from the pivotal Phase Ib/II FELIX study of obecabtagene autoleucel (obe-cel) in adults with relapsed or refractory B-cell ALL, at the 2024 ASCO Annual Meeting. The majority of responders exhibited durable responses, indicating the potential for long-term survival outcomes. At a median follow-up of 21.3 months, 40% of responders remained in remission without the need for stem cell transplant (SCT) or other therapy. Improved event-free survival was associated with the persistence of CAR T cells and B-cell aplasia, a condition where CD19-expressing B-lymphocytes are depleted. These promising results suggest obe-cel could become a standard-of-care treatment for relapsed or refractory B-ALL, offering new hope for patients with limited options.
CB-010 presents a novel approach to addressing aggressive relapsed or refractory B cell non-Hodgkin lymphoma (r/r B-NHL). During the ASCO Annual Meeting poster presentation, Caribou Biosciences unveiled fresh insights from the Phase I trial of CB-010 in second-line large B cell lymphoma (LBCL). The data suggests that a single dose of CB-010 holds promise in matching, or even surpassing, the safety, efficacy, and long-term response seen with approved autologous CAR-T cell therapies.
Further list would be provided in the report
CAR T-Cell Therapy for Non-Hodgkin's lymphoma Therapeutics Market Report Scope
- The CAR T-Cell Therapy for Non-Hodgkin's lymphoma therapeutics market report covers a segment of key events, an executive summary, and a descriptive overview, explaining its causes, signs, symptoms, pathogenesis, and currently used CAR-T therapies.
- Comprehensive insight into the epidemiology segments and forecasts, disease progression, and treatment guidelines has been provided.
- Additionally, an all-inclusive account of the emerging therapies and the elaborative profiles of late-stage and prominent therapies will impact the current treatment landscape.
- A detailed review of the CAR T-Cell Therapy for Non-Hodgkin's lymphoma therapeutics market, historical and forecasted CAR T-Cell Therapy for Non-Hodgkin's lymphoma market size, market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM therapy outreach.
- The CAR T-Cell Therapy for Non-Hodgkin's lymphoma therapeutics market report provides an edge while developing business strategies, by understanding trends, through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help shape and drive the 7MM of CAR T-Cell Therapy for Non-Hodgkin's lymphoma drugs market.
CAR T-Cell Therapy for Non-Hodgkin's lymphoma Therapeutics Market Report Insights
- Patient-based CAR T-Cell Therapy for Non-Hodgkin's lymphoma Market Forecasting
- Therapeutic Approaches
- CAR T-Cell Therapy for Non-Hodgkin's lymphoma Pipeline Drugs Analysis
- CAR T-Cell Therapy for Non-Hodgkin's lymphoma Market Size and Trends
- Existing and Future CAR T-Cell Therapy for Non-Hodgkin's lymphoma Drugs Market Opportunity
CAR T-Cell Therapy for Non-Hodgkin's lymphoma Therapeutics Market Report Key Strengths
- 11 Years CAR T-Cell Therapy for Non-Hodgkin's lymphoma Market Forecast
- The 7MM Coverage
- CAR T-Cell Therapy for Non-Hodgkin's lymphoma Epidemiology Segmentation
- Key Cross Competition
- Conjoint Analysis
- CAR T-Cell Therapy for Non-Hodgkin's lymphoma Drugs Uptake
- Key CAR T-Cell Therapy for Non-Hodgkin's lymphoma Market Forecast Assumptions
CAR T-Cell Therapy for Non-Hodgkin's lymphoma Therapeutics Market Report Assessment
- Current CAR T-Cell Therapy for Non-Hodgkin's lymphoma Treatment Market Practices
- CAR T-Cell Therapy for Non-Hodgkin's lymphoma Unmet Needs
- CAR T-Cell Therapy for Non-Hodgkin's lymphoma Pipeline Drugs Profiles
- CAR T-Cell Therapy for Non-Hodgkin's lymphoma Drugs Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
FAQs
- What was the CAR-T in NHL Market share (%) distribution in 2023, and what would it look like in 2034?
- At what CAGR is the CAR-T in NHL market expected to grow in the 7MM during the forecast period (2024–2034)?
- Among the emerging therapies, what potential therapies are expected to disrupt the CAR-T in NHL market?
- What will be the impact on the sales of YESCARTA after the entry of novel therapies in the market?
- What are the disease risks, burdens, and unmet needs of CAR-T in NHL? What will be the growth opportunities across the 7MM concerning the patient population with CAR-T in NHL?
- Which emerging therapy is going to garner the maximum market share in the 7MM in 2034?
- What are the various recent and upcoming events expected to improve the uptake of CAR-T in NHL?
- How much market share will be captured by CAR-Ts by 2034?
- What are the current options for the treatment of CAR-T in NHL? What are the current guidelines for treating NHL in the US, Europe, and Japan?
- What are the recent novel therapies, targets, mechanisms of action, and technologies being developed to overcome the limitations of existing therapies?
- What is the patient share in frontline transplant-eligible and transplant-ineligible?
- Which countries within EU4 and the UK do not have access to CAR-T therapies?
Reasons to Buy
- The CAR T-Cell Therapy for Non-Hodgkin's lymphoma therapeutics market report will help develop business strategies by understanding the latest trends and changing treatment dynamics driving the CAR-T in NHL.
- Insights on patient burden/disease, CAR T-Cell Therapy for Non-Hodgkin's lymphoma Incidence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years.
- Understand the existing CAR T-Cell Therapy for Non-Hodgkin's lymphoma Market opportunities in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
- Identifying strong upcoming players in the market will help devise strategies to help get ahead of competitors.
- To understand the future market competition in the CAR T-Cell Therapy for Non-Hodgkin's lymphoma Market.
- Highlights of access and reimbursement policies of current therapies, barriers to accessibility of expensive off-label therapies, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing market so that the upcoming players can strengthen their development and launch strategy.
Stay Updated with us for Recent Articles
-market.png&w=256&q=75)
.jpg)

.jpg)


-thumbnail.jpg&w=3840&q=75)
