Chronic Venous Insufficiency Market Summary
- The Chronic Venous Insufficiency market in the 7MM was USD 2,652 million in 2025 and projected to reach USD 5,951 million by 2034.
- The Chronic Venous Insufficiency market is projected to grow at a CAGR of 9.4% by 2034 in leading countries (US, EU4, UK and Japan).
Chronic Venous Insufficiency Market and Epidemiology Analysis
- CVI prevalence is higher in Western Europe than in other regions, but there are significant gaps in epidemiological data, with a reliance on outdated studies that do not reflect current trends. Moreover, large-scale comparative cohort studies are limited, particularly in Asian countries where research is sparse, hindering a comprehensive global understanding of prevalence and impact.
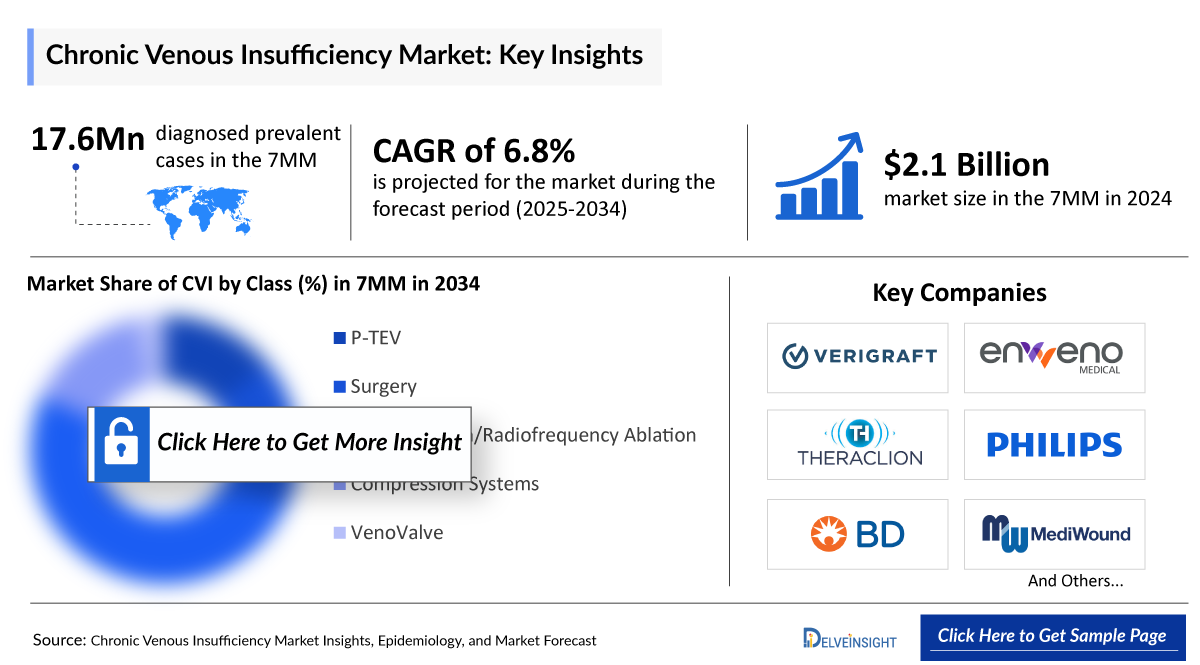
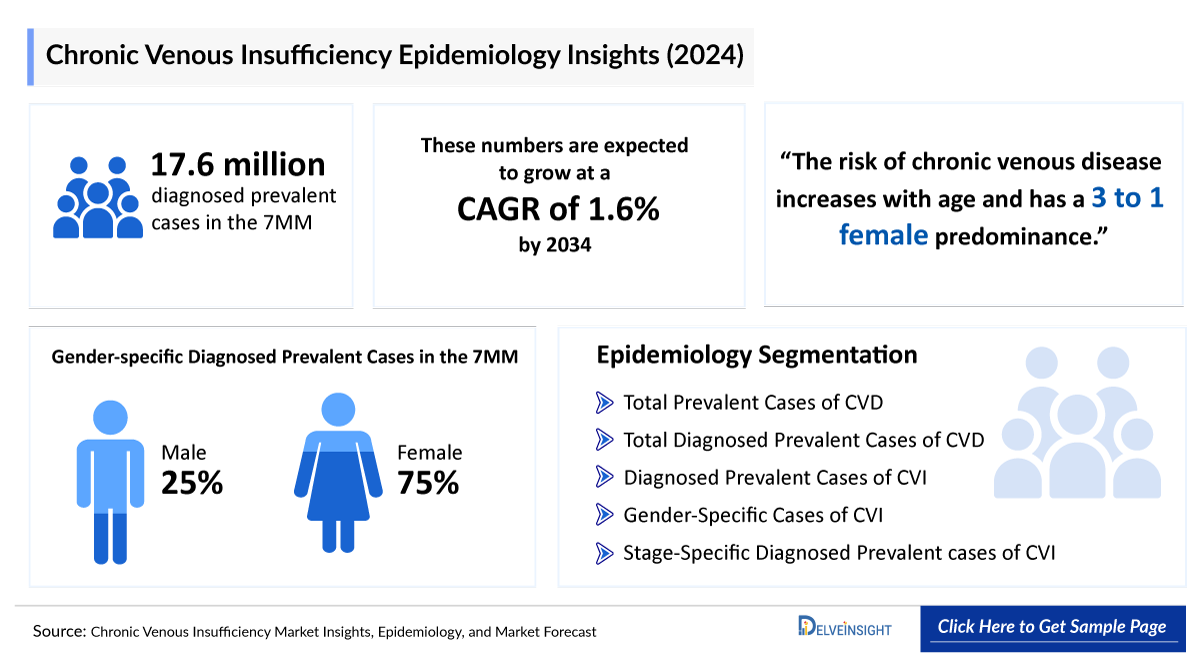
- In the 7MM, the total diagnosed prevalent cases of CVI were 17,641,600 approximately in 2020, which is expected to increase at a CAGR of 1.4% by 2034.
- The total market size of Chronic Venous Insufficiency in the 7MM was USD 2,370 million in 2020, which is anticipated to rise to USD 5,950 million by 2034 at a CAGR of 6.8%.
- In December 2023, the US Food and Drug Administration (FDA) approved the Duo Venous Stent System for the treatment of chronic venous Insufficiency.
- In the 7MM the US accounted for higher prevalance of 4900 cases of females over 1600 cases of males. These numbers are expected to increase during the forecast period (2025-2034).
- The total market size of Chronic Venous Insufficiency in Japan was USD 231 million in 2020, which is anticipated to rise to USD 298 million by 2034 at a CAGR of 1.8%.
- The diagnosis of CVI involves a combination of medical history, physical examination, and imaging tests. Along with this, some imaging tests are also recommended, such as duplex ultrasound, magnetic resonance venogram (MRV), CT venogram, and venogram. Additional tests, such as plethysmography and ambulatory venous pressure measurement, may be used to confirm the diagnosis and assess the severity of CVI.
- The treatment options for CVI include endovenous nonthermal ablation, sclerotherapy, radiofrequency ablation, laser ablation, and compression therapy. Additional therapies include balneotherapy for pain relief and plant-based extracts like Butcher's broom, horse chestnut, and red vine leaf. Improving patient compliance through more comfortable treatment options and lifestyle modifications remains a challenge.
Chronic Venous Insufficiency Market size and forecast
- 2025 Chronic Venous Insufficiency Market Size: USD 2652 million
- 2034 Projected Chronic Venous Insufficiency Market Size: USD 5951 million
- Chronic Venous Insufficiency CAGR (2025-2034): 9.4%
- Largest Chronic Venous Insufficiency Market: United States
Factors Impacting the Chronic Venous Insufficiency Market Growth
Rising CVI Prevalence
Based on DelveInsight's assessment in 2024, the 7MM had approximately 17 million diagnosed prevalent cases of CVI, which is expected to increase at a CAGR of 1.4% by 2034. These are expected to rise due to the growing geriatric population and advancements in diagnostic capabilities during the forecast period (2025−2034).
Innovations in Non-Pharmacological CVI Treatments
Advancements in technology, like advanced compression devices and therapeutic ultrasound, enhance non-pharmacological treatments for CVI by optimizing venous circulation and aiding tissue repair. These innovations offer precise and personalized care, improving treatment outcomes and patient satisfaction.
Key CVI Companies Driving R&D
The clinical trial landscape of CVI possesses potential candidates. Companies like VERIGRAFT (P-TEV), enVVeno Medical Corporation (VenoValve), Theraclion (SONOVEIN), MediWound (EscharEx), TR Therapeutics (TR987), and others are engaged in research and development efforts for CVI.
DelveInsight’s "Chronic Venous Insufficiency Market Insight, Epidemiology, and Market Forecast 2034" report delivers an in-depth understanding of the Chronic Venous Insufficiency historical and forecasted epidemiology as well as the Chronic Venous Insufficiency market trends in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
The Chronic Venous Insufficiency market report provides current treatment practices, emerging drugs/devices, Chronic Venous Insufficiency market share of individual therapies, and current and forecasted Chronic Venous Insufficiency market size from 2020 to 2034, segmented by seven major markets. The report also covers current Chronic Venous Insufficiency treatment practices/algorithms and unmet medical needs to curate the best of the opportunities and assess the underlying potential of the market.
Scope of the Chronic Venous Insufficiency Market | |
|
Study Period |
2020–2034 |
|
Forecast Period |
2025–2034 |
|
Geographies Covered |
US, EU4 (Germany, France, Italy, and Spain) the UK, and Japan |
|
Chronic Venous Insufficiency Epidemiology
|
Segmented by:
|
|
Chronic Venous Insufficiency key companies |
|
|
Chronic Venous Insufficiency key products |
|
|
Chronic Venous Insufficiency Market |
Segmented by:
|
|
Analysis |
|
Chronic Venous Insufficiency Disease Understanding and Treatment Algorithm
Chronic Venous Insufficiency Overview
CVD is a prevalent medical condition affecting the venous system, particularly the veins in the lower extremities. It encompasses a spectrum of venous disorders, ranging from mild cosmetic concerns like spider veins to severe and debilitating conditions like venous ulcers. CVI refers to the more advanced stages of CVD, which is a globally prevalent long-term condition in which the impaired blood flow in either superficial or deep veins, leads to increased venous pressure, known as venous hypertension. This condition manifests through various pathological changes, including swelling in the lower extremities, skin changes, and discomfort, all due to elevated venous pressure.
Chronic Venous Insufficiency Diagnosis
Diagnosing chronic venous insufficiency (CVI) relies on clinical features and diagnostic studies, with venous duplex ultrasound being the primary tool for providing detailed information on blood flow and venous anatomy. Clinical symptoms include edema, leg discomfort, varicose veins, and skin changes like hyperpigmentation and ulceration. Additional diagnostic techniques include phlebography, air plethysmography, phlebodynamometry, color-flow duplex ultrasound, and physical examination, all contributing to a comprehensive diagnosis. Differential diagnosis is essential to distinguish CVI from conditions like deep vein thrombosis, lymphedema, and systemic causes of edema, ensuring accurate treatment and preventing complications. A thorough medical history and physical examination are crucial for guiding appropriate treatment and improving patient outcomes.
Further details related to diagnosis will be provided in the report…
Chronic Venous Insufficiency Treatment
Treatment for chronic venous insufficiency (CVI) includes both conservative and invasive methods aimed at relieving discomfort, reducing swelling, stabilizing skin conditions, and promoting ulcer healing. Key treatments include endovenous ablation, sclerotherapy, radiofrequency ablation, and laser ablation, with compression therapy being central to improving venous return and reducing edema. However, patient adherence to compression garments is often poor due to discomfort. Pharmacological treatments involve venotonic agents (VADs), while plant-based extracts and balneotherapy are also used. Surgical options, such as vein repair and bypass, are considered for patients unresponsive to other treatments, particularly those with persistent symptoms or nonhealing ulcers.
Further details related to treatment will be provided in the report…
Chronic Venous Insufficiency Epidemiology
The Chronic Venous Insufficiency epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by the Total Prevalent Cases of CVD, Total Diagnosed Prevalent Cases for CVD, Stage-specific Diagnosed Prevalent Cases of CVD, Diagnosed Prevalent Cases of CVI, Gender-Specific Prevalent cases of Chronic Venous Insufficiency, and Total Treated Cases of Chronic Venous Insufficiency in the 7MM covering the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan from 2020 to 2034.
- In the US, there were 52,097,119 total diagnosed prevalent cases of CVD in 2020; which are expected to increase at a CAGR of 1.6% to 64,930,445 by 2034.
- In 2023 in the United States, there were more prevalent cases of CVI among females than number of male cases.
- In the US, CVI shows a marked gender disparity, with females accounting for 77.99% of cases compared to 22.01% in males.
Chronic Venous Insufficiency Recent Developments and Breakthroughs
- In September 2025, enVVeno Medical Corporation (Nasdaq: NVNO) announced plans to file a supervisory appeal of the not-approvable letter from the U.S. FDA’s Center for Devices and Radiological Health (CDRH). The letter, received on August 19, 2025, was in response to enVVeno's Premarket Approval (PMA) application for the VenoValve®, a surgical venous valve replacement designed to treat severe deep chronic venous insufficiency (CVI).
- In August 2025, enVVeno Medical Corporation announced that it received a not-approvable letter from the FDA regarding its Premarket Approval (PMA) application for VenoValve®, a surgical replacement venous valve for severe deep chronic venous insufficiency (CVI).
Chronic Venous Insufficiency Drug Chapters
The drug chapter segment of the Chronic Venous Insufficiency report encloses a detailed analysis of the marketed, early stage (I) pipeline drugs/devices. The marketed drugs/devices segment encloses products such as Duo Venous Stent System, Venclose System, and others. Furthermore, the current key players for emerging drugs/devices and their respective drug candidates include P-TEV by Verigraft AB, VenoValve by enVVeno Medical Corporation and others. The drug chapter also helps understand the Chronic Venous Insufficiency clinical trial details, expressive pharmacological action, agreements and collaborations, approval and patent details, and the latest news and press releases.
Chronic Venous Insufficiency Marketed Products
Duo Venous Stent System: Philips
The Duo Venous Stent System consists of a portfolio of self-expanding venous stent configurations mounted on disposable delivery systems for improving luminal diameter in symptomatic venous outflow obstructions. The portfolio approach includes delivery systems with either a hybrid venous stent implant (Duo Hybrid Stent) or an extension venous stent implant (Duo Extend Stent), enabling the clinician to custom tailor treatment in the iliofemoral venous anatomy based on disease patterns and severity. The Duo Venous Stent System is used to reopen parts of the iliofemoral veins that have narrowed after a blockage prevents blood flow (venous outflow obstruction). Limited blood flow in the iliofemoral veins may lead to swelling of the leg and pain when walking.
|
Product |
Company |
Approval Year (US) |
|
Duo Venous Stent System |
Philips |
December 2023 |
|
Venclose System |
BD |
December 2017 |
Chronic Venous Insufficiency Emerging Products
EscharEx: MediWound
EscharEx is a bromelain-based, bioactive enzymatic therapy developed by MediWound for the debridement of chronic and hard-to-heal wounds, such as venous leg ulcers (VLUs) and diabetic foot ulcers (DFUs). It is a topical, once-daily application consisting of a concentrate of proteolytic enzymes enriched in bromelain, derived from the stem of the pineapple plant. Its mechanism of action involves proteolytic enzymes that selectively cleave and remove necrotic tissue, eschar, and biofilm from the wound bed without damaging viable tissue, thereby preparing the wound for healing. This multimodal enzymatic debridement promotes granulation tissue formation, reduces bioburden and biofilm, and facilitates faster wound closure compared to standard care. EscharEx offers a non-surgical, rapid, and effective alternative to traditional surgical debridement, making it especially suitable for patients with chronic wounds and comorbidities. The drug is currently in Phase III of its clinical trials for the treatment of venous leg ulcers associated with chronic venous insufficiency.
P-TEV: Verigraft AB
P-TEV (Personalized Tissue-Engineered Veins) is an advanced therapy medicinal product (ATMP) being developed by Verigraft as an alternative to autologous or synthetic vascular grafts used in reconstructive vein surgery. The P-TEV drug substance consists of an extracellular matrix (ECM) scaffold in the form of a decellularized (DC) allogeneic vein scaffold which is populated with autologous components from the patient's own peripheral whole blood (PWB) in an ATMP manufacturing process performed under GMP. As the allogeneic immunogenic material has been removed from the donated vein segment by DC and as the perfusion uses autologous PWB, no immunosuppression is required.
Comparison of Chronic Venous Insufficiency Emerging Drugs | ||||
|
Emerging Drug |
Company |
Phase |
Molecule Type |
RoA |
|
EscharEx |
MediWound |
III |
Peptide hydrolase replacements |
Topical |
|
P-TEV |
Verigraft AB |
I |
Advanced Therapy Medicinal Product |
Implant |
|
VenoValve |
enVVeno Medical Corporation |
NA |
NA |
Implant |
Chronic Venous Insufficiency Market Outlook
- In the 7MM, the US accounted for the largest market size of Chronic Venous Insufficiency in 2023.
- The total market size in the US for Chronic Venous Insufficiency was estimated to be nearly USD 1,200 million in 2023, which is expected to grow during the forecast period (2025–2034).
- By 2034, among all the treatment, the highest revenue is expected to be generated by Laser Ablation/Radiofrequency Ablation, i.e., USD 1,787.9 million, while the lowest revenue is expected to be generated by TR987, i.e., USD 20.3 million in the 7MM.
Chronic venous insufficiency (CVI) is a condition where leg veins struggle to return blood to the heart, leading to blood pooling in the legs due to dysfunctional venous valves. Treatment varies based on severity and patient factors, starting with lifestyle changes and compression therapy, considered the gold standard. Minimally invasive procedures like Sclerotherapy, Endovenous Laser Therapy (EVLT), and Radiofrequency Ablation (RFA) are increasingly popular. New advancements include FDA-approved devices like the ClosureFast RFA catheter and the Philips Duo Venous Stent System, alongside emerging treatments like SONOVEIN and enVVeno Medical Corporation's VenoValve. The future of CVI treatment is promising, with innovations in minimally invasive techniques and biologic therapies enhancing patient outcomes.
Detailed market assessment will be provided in the final report.
Chronic Venous Insufficiency Drugs Uptake
This section focuses on the uptake rate of potential drugs expected to be launched in the market during 2025–2034, which depends on the competitive landscape, safety, and efficacy data along with order of entry. It is important to understand that the key players evaluating their novel therapies in the pivotal and confirmatory trials should remain vigilant when selecting appropriate comparators to stand the greatest chance of a positive opinion from regulatory bodies, leading to approval, smooth launch, and rapid uptake.
Chronic Venous Insufficiency Pipeline Development Activities
The report provides insights into therapeutic candidates in Phase I. It also analyzes key players involved in developing targeted therapeutics. Companies like Verigraft AB, Theraclion, enVVeno Medical Corporation, and others are actively engaging their product in research and development efforts for Chronic Venous Insufficiency. The pipeline of Chronic Venous Insufficiency possesses many potential drugs and there is a positive outlook for the therapeutics market, with expectations of growth during the forecast period (2025–2034).
Pipeline Development Activities
The report covers information on collaborations, acquisitions and mergers, licensing, and patent details for Chronic Venous Insufficiency emerging therapy.
Latest KOL Views on Chronic Venous Insufficiency
To keep up with current market trends, we take KOLs and SMEs’ opinions working in the domain through primary research to fill the data gaps and validate our secondary research. Industry Experts contacted for insights on the Chronic Venous Insufficiency evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake, along with challenges related to accessibility, including Medical Directors, Clinical Professors, and Dermatologists, and others.
DelveInsight’s analysts connected with 30+ KOLs to gather insights; however, interviews were conducted with 10+ KOLs in the 7MM. Centers such as the University of California,University of Michigan, Washington Hospital Healthcare System, University Hospital Münster, Germany etc., were contacted. Their opinion helps understand and validate current and emerging therapy treatment patterns or Chronic Venous Insufficiency market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
What KOLs are saying on Spinal Cord Injury Patient Trends?
- We believe the current landscape of CVI research has a notable absence of recent epidemiological data and new research publications. Many studies continue to rely on older information, highlighting a substantial gap in contemporary knowledge and insights into the condition. This lack of current research impedes progress in developing advanced treatment approaches and understanding emerging trends and risk factors associated with CVI
- Our estimates indicate that clinical determinants such as include age, female sex, arterial hypertension, obesity, smoking, and clinically overt cardiovascular disease contribute to CVI in Germany. These factors collectively increase the risk of developing CVI by influencing venous function, vascular health, and overall cardiovascular risk profile. Therefore, understanding and addressing these determinants are crucial for effective prevention and management strategies aimed at reducing the burden of CVI in the population
Chronic Venous Insufficiency Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the Analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving treatment landscape.
Chronic Venous Insufficiency Market Access and Reimbursement
The treatment and management of CVI are expensive. Botulinum toxin injections, commonly used to treat spasmodic torticollis, are costly and require frequent administration, leading to significant ongoing expenses for patients and healthcare systems. Effective reimbursement policies are crucial, as they can enhance treatment adherence, improve symptom control, and potentially reduce long-term healthcare costs by decreasing the need for additional medical services. To support patients, various organizations in the Seven Major Markets (7MM) offer programs like CMS coverage for botulinum toxin types A and B, the IPSEN CARES Patient Access Program, the BOTOX Savings Program, and the XEOMIN Savings Program. These initiatives help mitigate the financial burden and improve access to essential treatments.
Detailed market access and reimbursement assessment will be provided in the final report.
Scope of the Chronic Venous Insufficiency Market Report
- The report covers a segment of key events, an executive summary, and a descriptive overview of Chronic Venous Insufficiency, explaining its causes, signs, symptoms, pathogenesis, and currently used therapies.
- Comprehensive insight into the epidemiology segments and forecasts, disease progression, and treatment guidelines has been provided.
- Additionally, an all-inclusive account of the emerging therapies and the elaborative profiles of late-stage and prominent products will impact the current treatment landscape.
- A detailed review of the Chronic Venous Insufficiency market, historical and forecasted market size, market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The report provides an edge while developing business strategies by understanding trends through SWOT analysis and KOL views, patient journey, and treatment preferences that help shape and drive Chronic Venous Insufficiency.
Chronic Venous Insufficiency Market Report Insights
- Patient Population
- Therapeutic Approaches
- Chronic Venous Insufficiency Pipeline Analysis
- Chronic Venous Insufficiency Market Size and Trends
- Existing and Future Market Opportunity
Chronic Venous Insufficiency Market Report Key Strengths
- Eleven Years Forecast
- The 7MM Coverage
- Chronic Venous Insufficiency Epidemiology Segmentation
- Key Cross Competition
- Drugs Uptake and Key Market Forecast Assumptions
Chronic Venous Insufficiency Market Report Assessment
- Current Treatment Practices
- Unmet Needs
- Pipeline Product Profiles
- Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
Key Questions Answered in the Chronic Venous Insufficiency Report
- What was the Chronic Venous Insufficiency market size, the market size by therapies, market share (%) distribution in 2020, and what would it look like by 2034? What are the contributing factors for this growth?
- What can be the future treatment paradigm for Chronic Venous Insufficiency?
- What are the disease risks, burdens, and unmet needs of Chronic Venous Insufficiency? What will be the growth opportunities across the 7MM concerning the patient population with Chronic Venous Insufficiency?
- What are the current options for the treatment of Chronic Venous Insufficiency? What are the current guidelines for treating Chronic Venous Insufficiency in the 7MM?
- What are the recent novel therapies, targets, mechanisms of action, and technologies being developed to overcome the limitations of existing therapies?
- What is the patient share in Chronic Venous Insufficiency?
Reasons to Buy
- The report will help develop business strategies by understanding the latest trends and changing treatment dynamics driving the Chronic Venous Insufficiency market.
- Insights on patient burden/disease prevalence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years.
- Understand the existing market opportunities in varying geographies and the growth potential over the coming years.
- Identifying strong upcoming players in the market will help devise strategies to help get ahead of competitors.
- Highlights of access and reimbursement policies of current therapies, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing market so that the upcoming players can strengthen their development and launch strategy.