Factor Xa Inhibitor Market
- Factor Xa inhibitors market is projected to witness substantial growth in the coming years, driven by the rising number of diagnosed patients due to advancements in indications related to these therapies. This growth is further supported by increasing awareness of factor Xa and a growing pipeline of inhibitors currently in clinical trials or awaiting regulatory approval from various companies.
- Factor Xa inhibitors, a type of direct oral anticoagulant (DOAC), prevent blood clots by selectively blocking factor Xa, reducing thrombin production and interrupting the coagulation cascade.
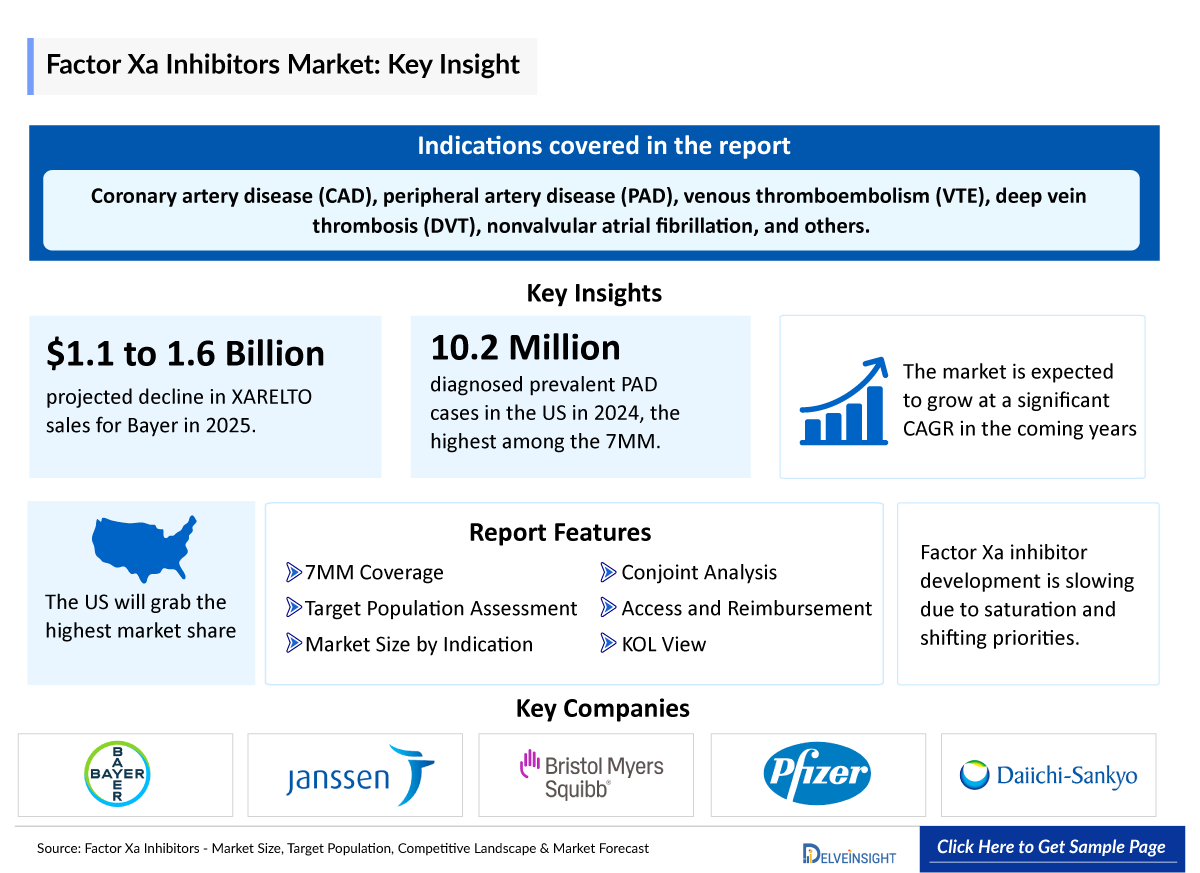
- Several Factor Xa inhibitors have been approved for clinical use, including XARELTO (rivaroxaban, Bayer and Janssen), ELIQUIS (apixaban, Bristol Myers Squibb and Pfizer), and SAVAYSA (edoxaban, Daiichi Sankyo).
- Factor Xa inhibitors have largely supplanted warfarin due to their predictable effects and reduced need for monitoring. Their shorter half-lives, compared to warfarin, allow drug levels to decrease more quickly upon discontinuation in patients with normal renal function, enhancing safety and flexibility in management.
- Factor Xa inhibitors are approved for preventing stroke and systemic embolism in nonvalvular atrial fibrillation, treating and preventing deep vein thrombosis (DVT) and pulmonary embolism, reducing venous thromboembolism (VTE) risk after orthopedic surgery, and lowering cardiovascular events in coronary artery disease (CAD) and peripheral artery disease (PAD), offering targeted and convenient anticoagulation.
- All Factor Xa inhibitors are contraindicated in active bleeding and carry US Black Box Warnings for spinal/epidural hematomas and increased thromboembolic risk with abrupt discontinuation. Careful transition to alternative anticoagulation is essential to prevent complications.
- In 2025, Bayer anticipates a significant decline in sales for its anticoagulant drug XARELTO, projecting a reduction of between USD 1.1 billion to USD 1.6 billion. The drop is attributed to the expiration of key patents, increased exposure to unfavorable EU patent rulings, and the emergence of at-risk generic launches.
- ELIQUIS’s Q1 2025 US sales demonstrated solid demand-driven growth; however, revenue gains were tempered by the financial impact of the Medicare Part D redesign, which introduced lower patient out-of-pocket costs and shifted a greater share of discounts to manufacturers.
- BEVYXXA (betrixaban), an anticoagulant developed by Portola Pharmaceuticals to prevent blood clots, was discontinued in April 2020 for business-related reasons.
- The development landscape for factor Xa inhibitors appears limited, suggesting a potential slowdown in innovation due to therapeutic saturation or shifting research priorities. This may restrict future treatment options and reduce flexibility in addressing emerging clinical needs.
- Ongoing development of specific reversal agents and exploration of combination or broad-spectrum anticoagulants highlight efforts to address current limitations and unmet needs in Factor Xa inhibitor therapy.
DelveInsight’s “Factor Xa Inhibitors Market Size, Target Population, Competitive Landscape, and Market Forecast – 2034” report delivers an in-depth understanding of the Factor Xa Inhibitors, historical and Competitive Landscape as well as the Factor Xa inhibitor market trends in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
The Factor Xa Inhibitors market report provides current treatment practices, market share of individual therapies, and current and forecasted 7MM Factor Xa Inhibitor market size from 2020 to 2034. The report also covers current Factor Xa Inhibitors treatment practices/algorithms and unmet medical needs to curate the best opportunities and assess the market’s potential.
|
Study Period |
2020–2034 |
|
Forecast Period |
2025–2034 |
|
Geographies Covered |
US, EU4 (Germany, France, Italy, and Spain) the UK, and Japan |
|
Factor Xa Inhibitors Epidemiology |
Segmented by:
|
|
Factor Xa Inhibitors Key Companies |
|
|
Factor Xa Inhibitors Key Therapies |
|
|
Factor Xa Inhibitors Market |
Segmented by:
|
|
Analysis |
|
Factor Xa Inhibitors Market: Understanding
Factor Xa Inhibitors Overview
Factor Xa inhibitors are anticoagulants used to treat and prevent blood clots in veins, and to prevent stroke and embolism in individuals with atrial fibrillation. Factor Xa, a serine protease, plays a crucial role in the coagulation pathway by cleaving prothrombin to generate thrombin, a key step in blood clot formation. Targeting factor Xa is considered advantageous for controlling thrombogenic events as it requires lower plasma levels of the inhibitor, potentially offering a better therapeutic index for anticoagulation with reduced risk of bleeding.
Inhibition of clotting factor Xa has been evaluated as a potential target for anticoagulation therapy with the hypothesis that using target-specific therapy will alleviate some of the dosing variability observed with the vitamin K antagonist. Three-factor Xa inhibitors are currently indicated for use in non-valvular atrial fibrillation. Similar to the vitamin K antagonist, warfarin, all of the factor Xa inhibitors are administered orally. The Factor Xa inhibitors have several approved indications for use including stroke or systemic embolism risk reduction in patients with non-valvular atrial fibrillation, treatment and reduction of recurrent deep vein thrombosis and pulmonary embolism, and VTE prophylaxis following knee or hip replacement surgery.
Further details related to country-based variations are provided in the report…
Factor Xa Inhibitors Epidemiology
The factor Xa inhibitors epidemiology chapter in the factor Xa inhibitors market report provides historical as well as forecasted epidemiology segmented as total cases in selected indications for factor Xa inhibitors, total eligible patient pool for factor Xa inhibitors in selected indications, total treated cases in selected indications for factor Xa inhibitors in the 7MM covering the United States, EU4 (Germany, France, Italy, and Spain), and the United Kingdom, and Japan from 2020 to 2034.
- Among 7MM, the United States accounts for the highest number of diagnosed prevalent PAD cases with approximately 10,199,700 in 2024.
- Among the EU4 and the UK, Germany had the highest diagnosed prevalent population of peripheral arterial disease, followed by Italy, while Spain had the lowest diagnosed prevalent population in 2024.
- According to the Centers for Disease Control and Prevention (CDC), VTE, or blood clots, impact as many as 900,000 individuals in the United States annually.
Factor Xa Inhibitors Drug Chapters
The drug chapter segment of the factor Xa inhibitors drugs market reports encloses a detailed analysis of factor Xa inhibitor-marketed drugs. It also helps understand the factor Xa inhibitor’s clinical trial details, expressive pharmacological action, agreements and collaborations, approval and patent details, advantages and disadvantages of each included drug and the latest news and press releases.
Marketed Factor Xa Inhibitors Drugs
SAVAYSA/LIXIANA (edoxaban): Daiichi Sankyo
SAVAYSA is an orally active anticoagulant that specifically, reversibly, and directly inhibits factor Xa, and is used to reduce the risk of stroke and systemic embolism in patients with non-valvular atrial fibrillation, as well as to treat venous thromboembolism, including deep vein thrombosis and pulmonary embolism. Edoxaban, marketed as LIXIANA in Japan, Europe, and Asia, and as SAVAYSA in the US that succeeded olmesartan following its loss of exclusivity; since its launch, the company has steadily increased market share, particularly in Japan, Europe, and Asia, and is now focusing on strengthening life-cycle management and enhancing product awareness.
In February 2025, LIXIANA was approved in Japan for the treatment of chronic thromboembolic pulmonary hypertension (CTEPH), expanding its therapeutic indications.
In June 2015, Daiichi Sankyo announced that the European Commission granted marketing authorisation for LIXIANA for the prevention of stroke and systemic embolism in adults with nonvalvular atrial fibrillation (NVAF)who have one or more risk factors, as well as for the treatment and prevention of recurrent deep vein thrombosis and pulmonary embolism.
LIXIANA was first approved in Japan in April 2011 for VTE prevention after major orthopedic surgery and later, in September 2014, for stroke prevention in NVAF and for the treatment and prevention of recurrent VTE.
ELIQUIS (apixaban): Bristol-Myers Squibb
ELIQUIS is an oral selective Factor Xa inhibitor. By inhibiting Factor Xa, a key blood clotting protein, Eliquis decreases thrombin generation and thus blood clot formation. ELIQUIS is a prescription medicine approved for multiple indications in the US, the European Union (which includes 28 member states plus Iceland and Norway) and Japan, as well as a number of other countries around the world based on efficacy and safety data, including results from seven Phase III clinical trials. In Japan, ELIQUIS is approved for the prevention of stroke and systemic embolism in patients with NVAF. ELIQUIS is also approved in Japan for the treatment of VTE, which includes DVT, and pulmonary embolism, and prevention of recurrent DVT and pulmonary embolism following initial therapy.
The company expects generic entry for ELIQUIS in the US starting April 2028, while in Europe, although patent challenges are ongoing in certain countries, key market patent expirations are anticipated in the second half of 2026.
|
Product |
Company |
RoA |
Approval |
Indication (US FDA) |
|
ELIQUIS (apixaban) |
Bristol-Myers Squibb |
Oral |
US: 2012 EU: 2012 JP: 2012 |
|
|
SAVAYSA/ LIXIANA (edoxaban) |
Daiichi Sankyo |
Oral |
US: 2015 EU: 2015 JP: 2014 |
|
|
XARELTO (rivaroxaban) |
Bayer |
Oral |
US: 2011 EU: 2008 JP: 2022 |
|
Note: Detailed current therapies assessment will be provided in the full report.
Factor Xa Inhibitors Market Outlook
Direct oral anticoagulants (DOACs) have replaced vitamin K antagonists for many indications. Currently, available DOACs include dabigatran, which inhibits thrombin, and apixaban, edoxaban, and rivaroxaban, which inhibit factor Xa. A new class of DOACs is under development. These new DOACs, which include asundexian and milvexian, inhibit FXIa, which is positioned in the intrinsic pathway of coagulation. Anticoagulants that target FXIa have the potential to be safer than the current DOACs because there is emerging evidence that FXI is essential for thrombosis but mostly dispensable for hemostasis. Hence there is almost no development in the field of Factor Xa Inhibitors.
Drug Class Insights
Factor Xa inhibitors are anticoagulant drugs that target and inhibit factor Xa, a crucial enzyme in the coagulation process. By blocking this enzyme, they prevent thrombin and fibrin clot formation, thereby lowering the risk of conditions like stroke and venous thromboembolism. Commonly used oral agents in this class include XARELTO, ELIQUIS, and SAVAYSA. These medications provide benefits such as consistent dosing, quick onset of action, and minimal dietary or drug interactions, making them favorable alternatives to warfarin. They are primarily prescribed for atrial fibrillation, deep vein thrombosis, and pulmonary embolism. However, they can be expensive and may require dosage adjustments in patients with impaired kidney function. In cases of major bleeding, reversal agents like andexanet alfa are available.
Factor Xa Inhibitors Market Outlook
The Factor Xa Inhibitors market is expected to grow significantly in the coming years. This is due to the increasing number of patients who are being diagnosed due to advancement in the indications related to factor Xa inhibitors. The growing awareness of factor Xa, and the increasing number of factor Xa inhibitors that are under clinical trials and filed for approval by various companies.
Currrently the Factor Xa Inhibitors market contain some of the approved Factor Xa Inhibitors therapies such as XARELTO, ELIQUIS, and SAVAYSA. Direct oral anticoagulants (DOACs) have replaced vitamin K antagonists for many indications. Currently, available DOACs include dabigatran, which inhibits thrombin, and apixaban, edoxaban, and rivaroxaban, which inhibit factor Xa. Factor Xa inhibitors offer significant advantages, including fixed oral dosing, fewer drug and food interactions, and lower risk of intracranial bleeding compared to warfarin, making them more convenient and safer for many patients. Their growing use in atrial fibrillation, DVT, and stroke prevention, supported by clinical guidelines and innovation in reversal agents, is driving market growth. However, challenges remain, including high drug and reversal costs, bleeding risks (especially gastrointestinal), and emerging competition from newer anticoagulant classes like factor XI inhibitors. Additionally, generic entry and access limitations in lower-income regions may impact Factor Xa Inhibitors market dynamics.
The withdrawal of BEVYXXA (betrixaban) negatively impacted market diversity and limited treatment options for extended VTE prophylaxis in hospitalized patients, highlighting the challenges of sustaining newer drugs in a highly competitive anticoagulant market.
Note: Detailed current therapies assessment will be provided in the full report of Factor Xa Inhibitors
Factor Xa Inhibitors Drugs Uptake
This section focuses on the uptake rate of potential approved drugs of factor Xa inhibitors.
Factor Xa Inhibitors Pipeline Development Activities
The Factor Xa Inhibitors drugs market report provides insights into different therapeutic approved candidates and analyzes key Factor Xa Inhibitors companies involved in developing targeted therapeutics.
The availability of numerous drugs is expected to create significant growth opportunities for the factor Xa inhibitor′s market over the forecast period.
Factor Xa Inhibitors Clinical Trials Activities
The Factor Xa Inhibitors market report covers information on collaborations, acquisitions and mergers, licensing, and patent details for factor Xa inhibitor therapies.
KOL Views on Factor Xa Inhibitors
To keep up with current and future Factor Xa Inhibitors market trends, we take Industry Experts’ opinions working in the domain through primary research to fill the data gaps and validate our secondary research. Industry experts were contacted for insights on factor Xa inhibitors' evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, drug uptake, along challenges related to accessibility.
DelveInsight’s analysts connected with 10+ KOLs to gather insights; however, interviews were conducted with 5+ KOLs in the 7MM. Centers such as Baptist Health Medical Group and others.
Their opinion helps understand and validate current and emerging therapy treatment patterns or factor Xa inhibitors market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the Factor Xa Inhibitors market and the unmet needs.
|
KOL Views |
|
“Currently available Factor Xa inhibitors have demonstrated similar efficacy and safety to warfarin in clinical trials, supporting their use as effective and reliable treatment options for patients with nonvalvular atrial fibrillation.” MD, Wingate University, US |
Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving treatment landscape.
Factor Xa Inhibitors Market Access and Reimbursement
Reimbursement may be referred to as the negotiation of a price between a manufacturer and payer that allows the manufacturer access to the Factor Xa Inhibitors market. It is provided to reduce the high costs and make the essential drugs affordable. Health technology assessment (HTA) plays an important role in reimbursement decision-making and recommending the use of a drug. These recommendations vary widely throughout the seven major markets, even for the same drug.
In the US healthcare system, both Public and Private health insurance coverage are included. Also, Medicare and Medicaid are the largest government-funded programs in the US. The major healthcare programs including Medicare, Medicaid, the Children's Health Insurance Program (CHIP), and the state and federal health insurance marketplaces are overseen by the Centers for Medicare & Medicaid Services (CMS). Other than these, Pharmacy Benefit Managers (PBMs), and third-party organizations that provide services, and educational programs to aid patients are also present.
The Factor Xa Inhibitors market report further provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of approved therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
The abstract list is not exhaustive, will be provided in the final report
Scope of the Factor Xa Inhibitors Market Report
- The Factor Xa Inhibitors market report covers a segment of key events, an executive summary, and a descriptive overview of the factor Xa inhibitors, explaining its mechanism, and therapies.
- Comprehensive insight into the competitive landscape, and forecasts, the future growth potential of treatment rate, drug uptake, and drug information have been provided.
- Additionally, an all-inclusive account of the therapies and the elaborative profiles of prominent therapies will impact the current landscape.
- A detailed review of the factor Xa inhibitors market, historical and forecasted market size, market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The Factor Xa Inhibitors market report provides an edge while developing business strategies, by understanding trends, through SWOT analysis, expert insights/KOL views, and treatment preferences that help shape and drive the 7MM factor Xa inhibitors market.
Factor Xa Inhibitors Market Report Insights
- Factor Xa Inhibitors Targeted Patient Pool
- Therapeutic Approaches
- Factor Xa Inhibitors Pipeline Analysis
- Factor Xa Inhibitors Market Size
- Factor Xa Inhibitors Market Trends
- Existing and future Factor Xa Inhibitors Market Opportunity
Factor Xa Inhibitors Market Report Key Strengths
- Ten years Forecast
- The 7MM Coverage
- Key Cross Competition
- Factor Xa Inhibitors Drugs Uptake
- Key Factor Xa Inhibitors Market Forecast Assumptions
Factor Xa Inhibitors Market Report Assessment
- Current Treatment Practices
- Factor Xa Inhibitors Unmet Needs
- Factor Xa Inhibitors Pipeline Product Profiles
- Factor Xa Inhibitors Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint)
Key Questions
- What was the factor Xa inhibitors total market size, the market size by therapies, market share (%) distribution, and what would it look like in 2034? What are the contributing factors for this growth?
- Which Factor Xa Inhibitors is going to be the largest contributor in 2034?
- Which is the most lucrative market for factor Xa inhibitors?
- What are the pricing variations among different geographies for approved therapies?
- How the reimbursement landscape has for factor Xa inhibitors evolved since the first one was approved? Do patients have any access issues that are driven by reimbursement decisions?
- What are the risks, burdens, and unmet needs of treatment with factor Xa inhibitors? What will be the growth opportunities across the 7MM for the patient population of factor Xa inhibitors?
- What are the key factors hampering the growth of the factor Xa inhibitors market?
- What key designations have been granted to the therapies for factor Xa inhibitors?
- What is the cost burden of approved Factor Xa Inhibitors therapies on the patient?
- Patient acceptability in terms of preferred therapy options as per real-world scenarios?
- What are the country-specific accessibility issues of expensive, recently approved Factor Xa Inhibitors therapies?
Reasons to buy
- The Factor Xa Inhibitors market report will help develop business strategies by understanding the latest trends and changing dynamics driving the factor Xa inhibitors market.
- Understand the existing Factor Xa Inhibitors market opportunities in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
- Identifying strong upcoming Factor Xa Inhibitors companies in the Factor Xa Inhibitors market will help devise strategies to help get ahead of competitors.
- Detailed analysis and ranking of indication-wise therapies under the conjoint analysis section to provide visibility around leading indications.
- Highlights of access and reimbursement policies of approved therapies, barriers to accessibility of expensive off-label therapies, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.