Venous Thromboembolism Market Summary
- The Venous Thromboembolism Market Size is anticipated to grow with a significant CAGR during the study period (2020-2034).
- The Venous Thromboembolism Market is anticipated to witness a substantial positive shift owing to better uptake of existing drugs, the expected market launch of one-time gene therapies, and raised awareness.
- The leading Venous Thromboembolism Companies such as Pfizer, Bristol-Myers Squibb, Bayer, Janssen LLC, Johnson & Johnson Pharma, Cristália Produtos Químicos Farmacêuticos Ltda., LEO Pharma, Sanofi, Daiichi Sankyo Inc., Astellas Pharma Inc., Boehringer Ingelheim, Regeneron Pharmaceuticals, and others.
Venous Thromboembolism Market & Epidemiology Analysis
- Venous Thromboembolism refers to thromboembolic events within veins, with Deep Vein Thrombosis (DVT) occurring in leg veins and Pulmonary Embolism in lung arteries.
- DVT involves blood clot formation in deeper leg veins, often post-major surgeries like knee or HIP Replacements. PE results from a clot blocking lung arteries, commonly originating in deep leg veins but can also form in arm or pelvic veins.
- Venous Thromboembolism affects more than 750,000 individuals each year across the European Union and is responsible for more than 370,000 Venous Thromboembolism-related deaths every year. Long-term complications of Venous Thromboembolism include post-thrombotic syndrome and chronic thromboembolic pulmonary hypertension.
- A history of previous Venous Thromboembolism events; surgery; medical conditions such as cancer or Spinal Cord Injury; pregnancy; paralysis or long periods of immobilization; specific genes; and certain circumstances related to age, race, and sex are all risk factors for Venous Thromboembolism.
- The leading risk factor for Venous Thromboembolism is long-term hospitalization, and the risk is high in patients with respiratory and circulatory system complications. The most common types of surgery associated with Venous Thromboembolism are orthopedic surgeries, especially knee and hip replacements.
- Active cancer accounts for almost 20% of all Venous Thromboembolism Incident Cases and is associated with poor outcomes, with around sixfold decreased survival rate, compared with cancer patients without Venous Thromboembolism.
- The signs and symptoms of Venous Thromboembolism differ from person to person. Venous Thromboembolism does not always cause symptoms until serious complications occur. Signs and symptoms of Venous Thromboembolism include acute onset of shortness of breath, Dyspnea, pleuritic chest pain, cough, or hemoptysis, complaints related to signs of DVT, lower-extremity swelling, and warmth to touch or tenderness.
- The Venous Thromboembolism Diagnosis is typically based on the medical history and assessment of risk for Venous Thromboembolism by gathering information and asking questions about certain factors. Based on these, tests such as the d-dimer test, compression ultrasound (CUS), venography, CT angiography, ventilation-perfusion (V/Q) scan, pulmonary angiography, and magnetic resonance imaging (MRI) are referred.
- Anticoagulant agents are essential for preventing and treating Venous Thromboembolism, reducing symptoms and recurrence risk. They include injectables like heparin or low molecular weight heparin (LMWH), and oral options like direct-acting oral anticoagulants (DOACs)- apixaban, dabigatran, rivaroxaban, edoxaban, and warfarin.
- The United States accounts for the largest Venous Thromboembolism Market Size, in comparison to EU4 (Germany, Spain, Italy, France), the United Kingdom, and Japan.
- Once anticoagulant therapy is stopped, up to 10% of people will experience a recurrence during the first year, associated with poor outcomes and significant mortality.
Request for unlocking the CAGR of the Venous Thromboembolism Treatment Market
Key Factors Driving the Venous Thromboembolism Market Growth
-
Rising Global Burden of VTE
The increasing incidence of deep vein thrombosis and pulmonary embolism, driven by aging populations, sedentary lifestyles, Obesity, and higher prevalence of chronic diseases, is significantly expanding the patient pool and driving demand for effective VTE therapies.
-
Growing Hospitalization and Surgical Procedures
The rise in major surgeries, trauma cases, cancer-related hospitalizations, and prolonged immobilization has increased the risk of VTE, thereby boosting the adoption of prophylactic and therapeutic anticoagulant treatments.
-
Advancements in Anticoagulant Therapies
The shift from conventional therapies to novel oral anticoagulants (NOACs/DOACs) with improved safety profiles, predictable dosing, and reduced need for monitoring is supporting wider market penetration and better patient compliance.
-
Improved Awareness and Diagnosis
Enhanced awareness among healthcare professionals and patients, along with improved diagnostic tools and screening practices, is leading to earlier detection and timely treatment of VTE, positively impacting market growth.
-
Expanding Indications and Long-Term Treatment Needs
Increasing use of anticoagulants for extended secondary prevention, recurrent VTE management, and in special populations such as cancer-associated thrombosis is contributing to sustained market expansion.
-
Strong Pipeline Activity and Innovation
Ongoing research and development focused on next-generation anticoagulants, safer long-term therapies, and agents with reduced bleeding risk is strengthening the competitive landscape and driving future growth opportunities.
-
Supportive Healthcare Policies and Reimbursement
Favorable treatment guidelines, inclusion of VTE prophylaxis in standard hospital care, and improving reimbursement frameworks in developed and emerging markets are facilitating broader access to VTE therapies.
-
Rising Focus on Personalized and Outpatient Care
The increasing preference for outpatient management of VTE, supported by oral therapies and personalized risk assessment strategies, is further accelerating market growth.DelveInsight's “Venous Thromboembolism Market Insights, Epidemiology and Market Forecast – 2034” report delivers an in-depth understanding of Venous Thromboembolism, historical and forecasted epidemiology as well as the Venous Thromboembolism therapeutics market trends in the United States, EU4 (Germany, Spain, Italy, and France), the United Kingdom, and Japan.
Venous Thromboembolism Treatment Market Report provides real-world prescription pattern analysis, emerging drugs, market share of individual therapies, and historical and forecasted 7MM Venous Thromboembolism market size from 2020 to 2034. The report also covers current Venous Thromboembolism treatment practices/algorithms and unmet medical needs to curate the best opportunities and assess the market’s underlying potential.
Scope of the Venous Thromboembolism Market Report | |
|
Study Period |
2020 to 2034 |
|
Forecast Period |
2024-2034 |
|
Geographies Covered |
|
|
Venous Thromboembolism Market |
|
|
Venous Thromboembolisms Market Size | |
|
Venous Thromboembolism Companies |
Pfizer, Bristol-Myers Squibb, Bayer, Janssen LLC, Johnson & Johnson Pharma, Cristália Produtos Químicos Farmacêuticos Ltd, LEO Pharma, Sanofi, Daiichi Sankyo Inc, Astellas Pharma Inc, Boehringer Ingelheim, Regeneron Pharmaceuticals, and others. |
|
Venous Thromboembolism Epidemiology Segmentation |
|
Venous Thromboembolism Understanding
Venous Thromboembolism Overview, Country-Specific Treatment Guidelines, and Diagnosis
Venous Thromboembolism encompasses Deep Vein Thrombosis (DVT) and Pulmonary Embolism (PE). DVT occurs when a clot forms in a deep vein, often in the lower leg, thigh, or pelvis, while PE happens when a clot dislodges and travels to the lungs. Venous Thromboembolism refers to any thromboembolic event within the venous system, typically caused by factors such as venous stasis, hypercoagulability, and endothelial damage, known as Virchow’s triad. The primary goal of testing for Venous Thromboembolism is to identify patients requiring anticoagulant therapy. Not all detected Venous Thromboembolism necessitate treatment; close monitoring with or without repeat imaging may suffice, particularly if thrombus extension is not observed. Diagnostic testing also aids in narrowing the differential diagnosis, crucial for patients with severe symptoms or signs compatible with other serious conditions.
Further details related to country-based variations in diagnosis are provided in the report...
Venous Thromboembolism Treatment
The therapy goal for Venous Thromboembolism is twofold: preventing thrombus extension and PE while alleviating short-term symptoms and averting recurrent events in the long term. Treatment typically comprises two phases: an initial 3-month active treatment phase followed by secondary prevention. Adequate anticoagulation during the initial phase is crucial, as it reduces the risk of embolization and death, with suboptimal anticoagulation linked to higher recurrence rates, especially within the first month. Therefore, anticoagulation forms the cornerstone of initial Venous Thromboembolism treatment.
Venous Thromboembolism Epidemiology
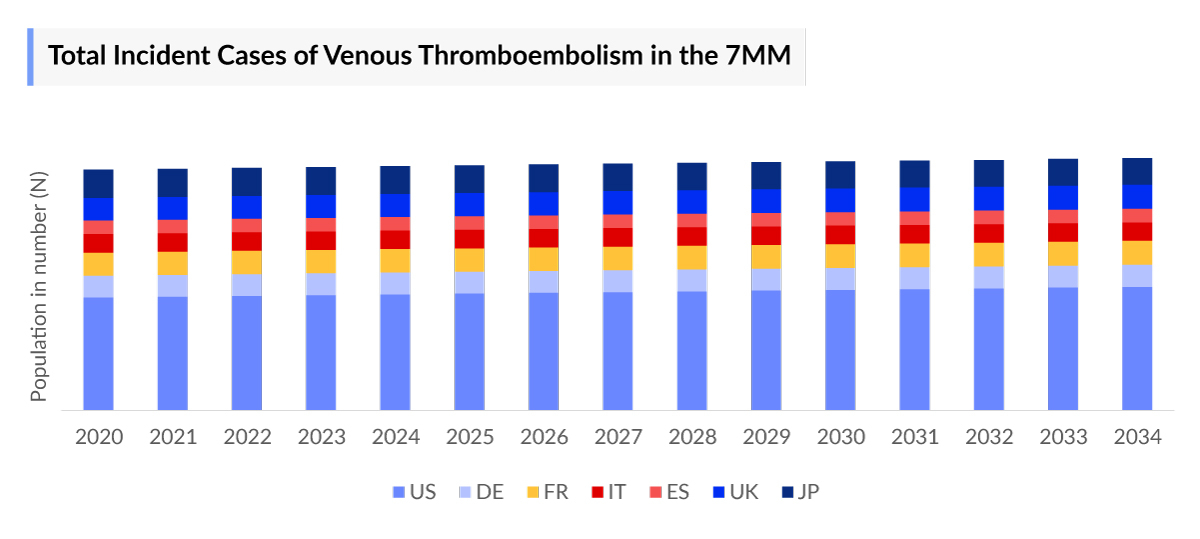
The Venous Thromboembolism epidemiology chapter in the report provides historical as well as forecasted in the 7MM covering the United States, EU4 countries (Germany, France, Italy, and Spain), the United Kingdom, and Japan from 2024 to 2034. The Venous Thromboembolism epidemiology is segmented with detailed insights into Total Incident Cases, Total Diagnosed Cases, Type-specific Cases, Occurrence-specific Cases, Total Age-specific Cases, Total Prophylactic/Preventive Setting Cases, Total Treated Cases, Total Potential (Mortality and Recurrent adjusted) Cases of Venous Thromboembolism.
Key findings from the Venous Thromboembolism Epidemiological Analyses and Forecast
- In EU4 countries and the UK, the highest number of Venous Thromboembolism Incident Cases was observed in France.
- In 2023, the incidence of Deep Vein Thrombosis cases in Venous Thromboembolism was higher compared to that of Pulmonary Embolism.
- The age group most impacted by Venous Thromboembolism comprises individuals =75 years of age.
Venous Thromboembolism Market Recent Developments
- In September 2025, Imperative Care received FDA 510(k) clearance for its Symphony® Thrombectomy System to treat pulmonary embolism (PE), expanding its use from venous thrombosis to cover the full spectrum of venous thromboembolism (VTE) patient care.
- In April 2025, Baebies received FDA Breakthrough Device Designation for its Anti-Factor Xa test on the FINDER® platform—the first point-of-care assay for heparin monitoring. The test delivers results in under 15 minutes from a small blood sample, supporting rapid and effective anticoagulant dose management in critical care.
Venous Thromboembolism Drug Analysis
The drug chapter segment of the Venous Thromboembolism Therapeutics Market Report encloses a detailed analysis of Venous Thromboembolism marketed drugs and late-stage (Phase III and Phase II) Venous Thromboembolism Pipeline Drugs. It also deep dives into the Venous Thromboembolism pivotal clinical trial details, recent and expected market approvals, patent details, the latest news, and recent deals and collaborations.
Venous Thromboembolism Marketed Drugs
-
LIXIANA (edoxaban): Daiichi Sankyo
Edoxaban is an oral Factor Xa Inhibitor used to reduce the risk of venous thrombosis, systemic embolization, and stroke in atrial fibrillation patients, and for treating DVT and PE to prevent thrombotic complications. While it may cause minimal serum aminotransferase elevations, clinically apparent acute liver injury hasn't been reported. Edoxaban works by blocking factor Xa, thereby reducing thrombin levels and preventing blood clot formation.
-
XARELTO (Rivaroxaban): Janssen Pharmaceuticals/ Bayer
The recommended dose is 10 mg of rivaroxaban taken orally once daily. The initial dose should be taken 6 to 10 hours after surgery, provided that hemostasis has been established. The duration of treatment depends on the individual risk of the patient for Venous Thromboembolism which is determined by the type of orthopedic surgery. The short duration of therapy (at least 3 months) should be considered in patients with DVT or PE provoked by major transient risk factors (i.e. recent major surgery or trauma). Longer duration of therapy should be considered in patients with provoked DVT or PE not related to major transient risk factors, unprovoked DVT or PE, or a history of recurrent DVT or PE.
Note: Detailed current therapies assessment will be provided in the full report of Venous Thromboembolism
Venous Thromboembolism Emerging Drugs
-
Abelacimab: Anthos Therapeutics
Abelacimab is a novel, highly selective, fully human monoclonal antibody that locks Factor XI in the inactive state, resulting in dual inhibitory activity against both Factor XI and its activated form, Factor XIa. Abelacimab is the only Factor XI inhibitor being studied for the prevention and treatment of both arterial and venous thromboembolic events. Abelacimab received a Fast Track Designation from the FDA in July 2022 for the treatment of thrombosis associated with cancer. In September 2022, abelacimab was also granted a Fast Track Designation for the prevention of stroke and systemic embolism in patients with atrial fibrillation.
-
BAY3018250: Bayer
BAY3018250 blocks the effect of a2ap, the main physiologic plasmin inhibitor in the body and an integral part of the blood clotting mechanism, helping to dissolve clots in situ. High levels of a2ap have been linked to poor outcomes in various cardiovascular diseases, including ischaemic stroke. Bayer is hoping that the antibody could provide an alternative to thrombolytics like t-PA, which are only moderately effective at dissolving blood clots in DVT. Thrombolytics frequently cause mild or moderate bleeding complications, and sometimes major complications, and are not suitable for every patient. The German group has started a Phase II trial – called SIRIUS – that will look at the effect of its anti-alpha2 antiplasmin (anti-a2ap) antibody BAY3018250 in patients with DVT, which occurs when a blood clot forms in one or more of the deep veins in the limbs, usually the legs.
Venous Thromboembolism Market Outlook
The leading Venous Thromboembolism Companies, such as Janssen Pharmaceuticals, Bayer, Daiichi Sankyo, and others, are evaluating their lead candidates in different stages of clinical development, respectively. They aim to investigate their products for the Venous Thromboembolism Treatment. Venous Thromboembolism Market Growth is expected to be primarily driven by the increased patient pool, especially in the prophylactic setting, expected improvement in the diagnosis rate owing to guidelines recommendations and government initiatives, and various advantages of DOACs, which will lead to an increased usage of this class in the upcoming years.
The prophylactic setting is the major contributor to the Venous Thromboembolism Market Size and contributes more than 90% of the Venous Thromboembolism Market due to a much higher patient pool than the treatment setting. Entry of biosimilars or generics of LMWH and DOACs, lack of public awareness regarding Venous Thromboembolism and inadequate management of Venous Thromboembolism in cancer and critically ill patients, risk of bleeding with anticoagulant treatment, complications of Venous Thromboembolism, and potential barriers to prophylaxis will pose hurdles to the Venous Thromboembolism Market growth.
Venous Thromboembolism Drugs Uptake
This section focuses on the uptake rate of potential Venous Thromboembolism drugs expected to be launched in the market during 2024–2034, which depends on the competitive landscape, safety, and efficacy data along with order of entry. It is important to understand that the key Venous Thromboembolism Companies evaluating their novel therapies in the pivotal and confirmatory trials should remain vigilant when selecting appropriate comparators to stand the greatest chance of a positive opinion from regulatory bodies, leading to approval, smooth launch, and rapid uptake.
Venous Thromboembolism Pipeline Development Activities
The Venous Thromboembolism Therapeutics Market Report provides insights into clinical trials within the Phase III and Phase II stages. It also analyzes key Venous Thromboembolism Companies involved in developing targeted therapeutics. The report covers information on collaborations, acquisitions and mergers, licensing, and patent details for Venous Thromboembolism emerging therapies.
Latest KOL Views on Venous Thromboembolism
To keep up with the real-world scenario in current and emerging market trends, we take opinions from Key Industry leaders working in the domain through primary research to fill the data gaps and validate our secondary research. Industry Experts were contacted for insights on the evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake along with challenges related to accessibility.
DelveInsight’s analysts connected with 20+ KOLs to gather insights; however, interviews were conducted with 5+ KOLs in the 7MM. Their opinion helps understand and validate current and emerging treatment patterns of Venous Thromboembolism. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
Venous Thromboembolism Qualitative Analysis Report
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT analysis and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of gaps in disease diagnosis, patient awareness, physician acceptability, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. Conjoint Analysis analyzes multiple approved and emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy.
Further, the therapies’ safety is evaluated wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Venous Thromboembolism Market Access and Reimbursement
The report further provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of currently used therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Scope of the Venous Thromboembolism Market Report
- The Venous Thromboembolism Market Report covers a segment of key events, an executive summary, a descriptive overview, explaining its causes, signs and symptoms, pathogenesis, and currently available therapies.
- Comprehensive insight has been provided into the epidemiology segments and forecasts, the future growth potential of the diagnosis rate, and disease progression along with country-specific Venous Thromboembolism Treatment guidelines.
- Additionally, an all-inclusive account of both the current and emerging therapies, along with the elaborate profiles of late-stage and prominent therapies, will have an impact on the current Venous Thromboembolism Treatment Market Landscape.
- A detailed review of the Venous Thromboembolism Treatment Market, historical and forecasted Venous Thromboembolism Market Size, Venous Thromboembolism Market Share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The Venous Thromboembolism Therapeutics Market Report provides an edge while developing business strategies, by understanding trends, through SWOT analysis and Expert Insights/KOL views, patient journey, and treatment preferences that help in shaping and driving the 7MM Venous Thromboembolism Drugs Market.
Venous Thromboembolism Market Report Insights
- Patient-based Venous Thromboembolism Market Forecasting
- Therapeutic Approaches
- Venous Thromboembolism Pipeline Analysis
- Venous Thromboembolism Market Size and Trends
- Existing and future Market Opportunity
Venous Thromboembolism Market Report Key Strengths
- 11 Years Venous Thromboembolism Market Forecast
- 7MM Coverage
- Venous Thromboembolism Epidemiology Segmentation
- Inclusion of country-specific Venous Thromboembolism Treatment guidelines
- KOL’s feedback on approved and emerging therapies
- Key Cross Competition
- Conjoint analysis
- Drugs Uptake and Key Market Forecast Assumptions
Venous Thromboembolism Market Report Assessment
- Current Venous Thromboembolism Treatment Practices
- Venous Thromboembolism Unmet Needs
- Venous Thromboembolism Pipeline Drugs Profiles
- Venous Thromboembolism Drugs Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
Key Questions Answered in the Venous Thromboembolism Report
Venous Thromboembolism Market Insights
- What is the growth rate of the 7MM Venous Thromboembolism treatment market?
- What was the Venous Thromboembolism Market Size, the market size by therapies, Venous Thromboembolism Market Share (%) distribution in 2020, and what would it look like in 2034? What are the contributing factors/key catalysts for this growth?
- Is there any unexplored patient setting that can open the window for growth in the future?
- What are the pricing variations among different geographies for approved and off-label therapies?
- How would the market drivers, barriers, and future opportunities affect the market dynamics and subsequent analysis of the associated trends? Although multiple expert guidelines recommend testing for targetable mutations before therapy initiation, why do barriers to testing remain high?
- What are the current and emerging options for the treatment of Venous Thromboembolism?
- How many companies are developing therapies for the treatment of Venous Thromboembolism?
- What are the recent novel therapies, targets, Venous Thromboembolism Mechanisms of Action, and technologies developed to overcome the limitations of existing therapies?
- Patient/physician acceptability in terms of preferred treatment options as per real-world scenarios?
- What are the country-specific accessibility issues of expensive, recently approved therapies?
Reasons to Buy the Venous Thromboembolism Market Report
- The Venous Thromboembolism Therapeutics Market Report will help in developing business strategies by understanding the latest trends and changing treatment dynamics driving the Venous Thromboembolism Market.
- Insights on patient burden/disease Venous Thromboembolism Prevalence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years
- Understand the existing market opportunities in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
- Identifying strong upcoming players in the Venous Thromboembolism Drugs Market will help devise strategies to help get ahead of competitors.
- Detailed analysis and ranking of class-wise potential current and emerging therapies under the conjoint analysis section to provide visibility around leading classes.
- Highlights of access and reimbursement policies of approved therapies, barriers to accessibility of expensive off-label therapies, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights into the unmet needs of the existing Venous Thromboembolism Drugs Market so that the upcoming players can strengthen their development and launch strategy.
Stay updated with us for New Articles:-

.jpg)

.jpg)




