Gastrointestinal Stromal Tumor Market
- The Gastrointestinal Stromal Tumor Therapeutics Market Size in the 7MM was around USD 450 million in 2023.
- The United States accounts for the highest GIST Market Size approximately 69% of the total GIST Market Size in 7MM in 2023, in comparison to the other major markets i.e., EU4 countries (Germany, Italy, France, and Spain) and the United Kingdom, and Japan.
- Among the EU4 and the UK, Germany had the highest Gastrointestinal Stromal Tumor Therapeutics Market Size with USD 33 million in 2023, while Spain had the lest market size of GIST with about USD 17 million in 2023.
- During the study period from 2020 to 2034, the United States consistently had the highest Gastrointestinal Stromal Tumor Incidence compared to the other 7MM countries. Of the approximately 16 thousand GIST Incident Cases estimated in the 7MM, around 43% originated from the US.
- Our assessments indicate that KIT and PDGFRA mutations are the most common events in GIST Pathogenesis, with BRAF mutations also affecting a portion of the population. In 2023, the US had around 80% cases of KIT mutation, 8% cases of PDGFRA mutation, 1% cases of BRAF mutation, and 11% cases of other mutations.
- KIT mutations are more prevalent in Gastrointestinal Stromal Tumor Pathogenesis due to the pivotal role of the KIT gene in regulating cell growth and survival. Mutations in KIT lead to dysregulated cellular processes, contributing to the development and aggressiveness of GIST.
- According to our assessments, the Gastrointestinal Stromal Tumor Incidence Population is categorized based on disease stage, including localized, regional, distant, and unknown stages. The majority of cases are observed in the localized stage.
- AYVAKIT (avapritinib) stands as the sole approved therapy for treating PDGFRA mutation-positive Gastrointestinal Stromal Tumor.
- The Gastrointestinal Stromal Tumor Therapeutics Market is currently driven by approved therapies across multiple lines of treatment. With a rising Gastrointestinal Stromal Tumor Incidence Cases and the anticipated launch of emerging therapies such as crenolanib, bezuclastinib, and others, the overall market size of GIST is expected to surge in the US from 2020 to 2034.
Request for Unlocking the Sample Page of the "Gastrointestinal Stromal Tumor Treatment Market"
DelveInsight’s report titled “Gastrointestinal Stromal Tumor Therapeutics Market Insights, Epidemiology, and Market Forecast – 2034” comprehensively analyzes Gastrointestinal Stromal Tumor. The report provides a comprehensive analysis of historical and projected epidemiological data, covering Total Gastrointestinal Stromal Tumor Incident Cases, Gastrointestinal Stromal Tumor Gender-specific Incident Cases, Gastrointestinal Stromal Tumor Age-specific Incident Cases, Gastrointestinal Stromal Tumor Mutation-specific Incident Cases, and Gastrointestinal Stromal Tumor Stage-specific Incident Cases.
The Gastrointestinal Stromal Tumor Therapeutics Market Report offers a comprehensive understanding of various aspects related to the Gastrointestinal Stromal Tumor patient population, including diagnosis, prescribing patterns, physician perspectives, market accessibility, therapies, and upcoming market developments across seven key markets: the United States, EU4 (Germany, France, Italy, and Spain), the UK, and Japan, covering the period from 2020 to 2034. The report evaluates existing treatment methods and algorithms for Gastrointestinal Stromal Tumor, analyzing the overall market potential, identifying business opportunities, and addressing significant unmet medical needs.
|
Study Period |
2020 to 2034 |
|
Forecast Period |
2024-2034 |
|
Geographies Covered |
|
|
Gastrointestinal Stromal Tumor Market |
|
|
Gastrointestinal Stromal Tumor Market Size | |
|
Gastrointestinal Stromal Tumor Companies |
Jiangsu Hengrui Medicine, Daiichi Sankyo Company, Cogent Biosciences, Advenchen Laboratories, AB Science, Immunicum AB, Novartis, Bristol-Myers Squibb, Hanmi Pharmaceutical Company Limited, Ascentage Pharma, Array BioPharma, Plexxikon, Arog Pharmaceuticals, Xencor Inc., DNAtrix Inc., Onyx Pharmaceuticals, Exelixis, Allarity Therapeutics, Theseus Pharmaceuticals, IDRx Inc., Allarity Therapeutics, and others. |
|
Gastrointestinal Stromal Tumor Epidemiology Segmentation |
|
Gastrointestinal Stromal Tumor Treatment Market
GIST diagnosis typically involves imaging studies like CT scans or endoscopy, followed by a biopsy to confirm the presence of GIST cells. However, limitations in diagnosis may arise due to the heterogeneous nature of GIST and the need for specialized expertise in interpreting biopsy results accurately. Gastrointestinal Stromal Tumor Treatment algorithms often begin with surgical resection for localized disease, followed by targeted therapy with tyrosine kinase inhibitors like imatinib for advanced or metastatic cases. Regular monitoring for treatment response and disease progression is essential, with adjustments made based on individual patient responses and genetic testing results.
The patient journey in GIST involves initial diagnosis, often prompted by symptoms like abdominal pain or gastrointestinal bleeding, followed by consultations with specialists for further evaluation and treatment planning. Treatment may involve a multidisciplinary approach, including surgery, medical oncology, and supportive care, with ongoing monitoring and management to optimize patient outcomes and quality of life.
Gastrointestinal Stromal Tumor Overview
Gastrointestinal Stromal Tumor is a rare cancer affecting the digestive tract or adjacent structures within the abdomen, typically arising from interstitial cells of Cajal (ICCs) or less differentiated precursor cells. Mutations in KIT or PDGFRA genes, or other rare gene alterations, drive GIST progression, with the majority of tumors exhibiting mutations in the KIT gene. A subset of GIST, known as wild-type GIST, lacks mutations in KIT or PDGFRA and may instead involve genes related to the succinate dehydrogenase (SDH) enzyme complex or other genes such as BRAF, KRAS, and NRAS. Understanding the molecular underpinnings of GIST, including its diverse genetic mutations, is crucial for advancing targeted therapies and improving patient outcomes.
Gastrointestinal Stromal Tumor Epidemiology
The epidemiology section on Gastrointestinal Stromal Tumor provides an analysis of historical and current patient populations, as well as projected trends across seven major countries (7MM). Utilizing insights from various studies and expert perspectives, it seeks to clarify the factors influencing current and anticipated patterns. Moreover, this part of the report presents information on the diagnosed patient population, emphasizing trends and fundamental assumptions.
Key Findings
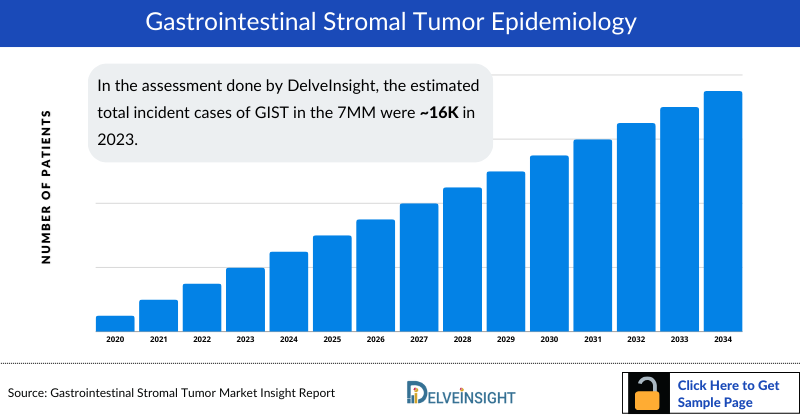
- In the assessment done by DelveInsight, the estimated total Gastrointestinal Stromal Tumor Incidence Cases in the 7MM were nearly 16 thousand in 2023.
- The highest Gastrointestinal Stromal Tumor Incidence Cases were accounted by the US in 2023 (approximately 7k cases), which are expected to show a steep rise soon due to the improvement in diagnostic testing and advancements in genetic testing and accounts for 43% of the total cases in the 7MM.
- In 2023, the Gastrointestinal Stromal Tumor Age-specific incident Cases accounted highest in the age group 66-80 years with nearly 6 thousand cases in the 7MM, followed by age group 51-65 years with around 5 thousand cases. While the least number of cases were seen in the age group 0-17 years.
- Among the European countries, Germany had the highest Gastrointestinal Stromal Tumor Incident Population with nearly 2 thousand cases, followed by Italy, which had incident population of over 1k in 2023. On the other hand, Spain had the lowest Gastrointestinal Stromal Tumor Incident Population.
- Japan had more than 2,500 GIST Incident cases for in 2023, accounting for approximately 16% of total Gastrointestinal Stromal Tumor Incident Cases in the 7MM.
- In 2023, our estimations indicate that within the 7MM, a higher percentage of males (52%) were affected by Gastrointestinal Stromal Tumor compared to females (48%). The reason for this gender difference could be attributed to various factors, including potential differences in genetic susceptibility, hormonal influences, occupational exposures, lifestyle habits, and healthcare-seeking behaviors.
Gastrointestinal Stromal Tumor Recent Developments
- In March 2025, Zai Lab (Shanghai) Co., Ltd. announced the initiation of a study aimed at evaluating the progression-free survival (PFS) of DCC-2618 in patients with advanced gastrointestinal stromal tumors (GIST) who have experienced disease progression following prior anticancer therapies. The assessment is based on an independent radiologic review.
- In March 2025, Deciphera Pharmaceuticals, LLC initiated a study to compare the efficacy of ripretinib versus sunitinib in patients with GIST who have progressed on or were intolerant to first-line imatinib therapy. The trial plans to randomize approximately 426 patients in a 1:1 ratio to receive either ripretinib (150 mg once daily, continuous dosing in 6-week cycles) or sunitinib (50 mg once daily, administered in 6-week cycles with 4 weeks on and 2 weeks off).
- In March 2025, Merck Sharp & Dohme LLC launched a study to assess the efficacy and safety of belzutifan monotherapy in participants with advanced pheochromocytoma/paraganglioma (PPGL), pancreatic neuroendocrine tumors (pNET), von Hippel-Lindau (VHL) disease-associated tumors, advanced wild-type gastrointestinal stromal tumors (wt GIST), or advanced solid tumors harboring hypoxia-inducible factor-2 alpha (HIF-2α) related genetic alterations. The primary objective is to determine the objective response rate (ORR) based on RECIST v1.1, as evaluated by blinded independent central review (BICR).
- In March 2025, Bayer conducted a Phase 2 open-label basket study to evaluate the efficacy and safety of orally administered reversible tyrosine kinase inhibitor BAY 2927088 in patients with metastatic or unresectable solid tumors harboring HER2-activating mutations.
Gastrointestinal Stromal Tumor Drugs Market Chapters
The drug chapter segment of the Gastrointestinal Stromal Tumor Therapeutics Market Report encloses the detailed analysis of Gastrointestinal Stromal Tumor marketed drugs, mid-phase, and late-stage Gastrointestinal Stromal Tumor Pipeline Drugs analysis. It also helps to understand the Gastrointestinal Stromal Tumor Clinical Trials details, expressive pharmacological action, agreements and collaborations, approval, and patent details of each included drug, and the latest news and press releases.
Gastrointestinal Stromal Tumor Marketed Therapies
- Ayvakit (Avapritinib): Blueprint Medicines Corporation
Ayvakit is a kinase inhibitor indicated for the treatment of adults with unresectable or metastatic GIST harboring a platelet-derived growth factor receptor alpha (PDGFRA) exon 18 mutation, including PDGFRA D842V mutations and Advanced Systemic Mastocytosis (AdvSM). The FDA granted full approval to Ayvakit based on efficacy results from the Phase I NAVIGATOR Gastrointestinal Stromal Tumor Clinical Trials, as well as combined safety results from multiple Gastrointestinal Stromal Tumor Clinical Trials for avapritinib.
- STIVARGA (Regorafenib): Bayer
Stivarga (BAY 73-4506) is the brand name for the generic chemotherapy agent regorafenib, an oral anticancer medication approved by the FDA for individuals with locally advanced, unresectable, or metastatic GIST who have previously received imatinib mesylate and sunitinib malate. In September 2021, Bayer presented study results indicating close proximity to the prespecified activity threshold, possibly influenced by a low recruitment rate. The notable frequency of undetected mutant GIST by Sanger sequencing underscores the importance of next-generation sequencing (NGS) in presumed KIT/PDGFR wild-type GIST.
Gastrointestinal Stromal Tumor Emerging Therapies
Crenolanib: Arog Pharmaceuticals
Crenolanib, an investigational small molecule drug, is currently undergoing Phase III Gastrointestinal Stromal Tumor Clinical Trials to assess its safety and efficacy in treating Acute Myeloid Leukemia (AML) and Gastrointestinal Stromal Tumor. Acting as a potent inhibitor of both wild-type and mutant forms of FLT3 (FMS-like Tyrosine Kinase 3) and PDGFRα/β (Platelet-derived Growth Factor Receptor), Crenolanib holds promise as a potential additional treatment option for patients with advanced or metastatic GIST harboring a D842V mutation in the PDGFRA gene, offering hope for improved outcomes in this specific subgroup.
Bezuclastinib (CGT9486/PLX9486): Cogent Biosciences, Inc. / Plexxikon Inc.
Bezuclastinib (CGT9486) is a highly selective Type I inhibitor of KIT receptor tyrosine kinase, specifically targeting oncogenic KIT with mutations in the activation loop encoded by exons 17 and 18, including the pivotal KIT D816V mutation found in the majority of patients with systemic mastocytosis. The combination of bezuclastinib with sunitinib, a Type-II inhibitor effective against KIT ATP-binding pocket mutations, demonstrated prolonged progression-free survival in extensively treated patients with gastrointestinal stromal tumor. Cogent Biosciences, Inc. holds worldwide rights to bezuclastinib, encompassing all facets of development, manufacturing, and commercialization activities related to this promising molecule.
|
Drug |
MoA |
RoA |
Company |
Phase |
|
Crenolanib |
Selective tyrosine kinase inhibitor (TKI) |
Oral |
Arog Pharmaceuticals |
III |
|
Bezuclastinib |
KIT inhibitor |
Oral |
Cogent Biosciences, Inc. / Plexxikon Inc. |
II/III |
|
XXX |
HSP90 inhibitor |
XX |
XXX |
I |
Gastrointestinal Stromal Tumor Market Outlook
Knowledge about the underlying genetic alterations revealed possible targeted treatment with TKIs such as imatinib and sunitinib. Continuous research efforts helped to further elucidate molecular insights of this disease and allowed the development of new treatment options based on the underlying molecular signature. Currently, there are seven therapies approved for GIST that includes five TKIs, and two NTRK inhibitors such as GLEEVEC (Imatinib Mesylate), STIVARGA (Regorafenib), SUTENT (sunitinib malate), AYVAKIT (avapritinib), QINLOCK (Ripretinib), ROZLYTREK (Entrectinib), and VITRAKVI (Larotrectinib), approved in different lines. Along with these, there are certain therapies being used as off-label therapies in patients resistant to approved therapies.
Targeted therapy with imatinib, an oral, small molecule, selective tyrosine-kinase inhibitor of KIT, PDGFRA, and ABL that was approved for first-line treatment for advanced GIST in 2002, for adjuvant treatment in high-risk patients in 2008, and for use in adult patients following surgical removal of CD117-positive GIST in 2012 by USFDA (the United States Food and Drug Administration), in 2001 by EMA (European Medicines Agency), and 2003 by PMDA (Pharmaceuticals and Medical Devices Agency), revolutionized treatment for GIST.
Though the management of GIST was transformed after the arrival of imatinib which became the standard first-line treatment for metastatic GIST. On the other hand, despite a clinical benefit rate of 80%, half of those having a clinical benefit eventually progress after 2 years of therapy due to acquired imatinib resistance.
Moreover, for the subset of patients with KIT/PDGFRA-wild type GISTs, there are significant unmet needs present that includes SDH-negative/deficient GIST, NF1-mutant GIST both sporadic and syndromic (associated with type I neurofibromatosis), RAS-mutant, FGFR mutant GISTs and others. These groups of patients exhibit sensitivity to other inhibitors (except TKIs) and thus shall be considered differently; therapeutics development shall be tailored to their particular genetic defects and potential molecular vulnerabilities.
Gastrointestinal Stromal Tumor Market Segmentation
DelveInsight’s ‘Gastrointestinal Stromal Tumor Therapeutics Market Insights, Epidemiology, and Market Forecast – 2034’ report provides a detailed outlook of the current and future Gastrointestinal Stromal Tumor market, segmented within countries and by therapies. Further, the market of each region is then segmented by each therapy to provide a detailed view of the current and future market share of all therapies.
Gastrointestinal Stromal Tumor Therapeutics Market Size by Countries
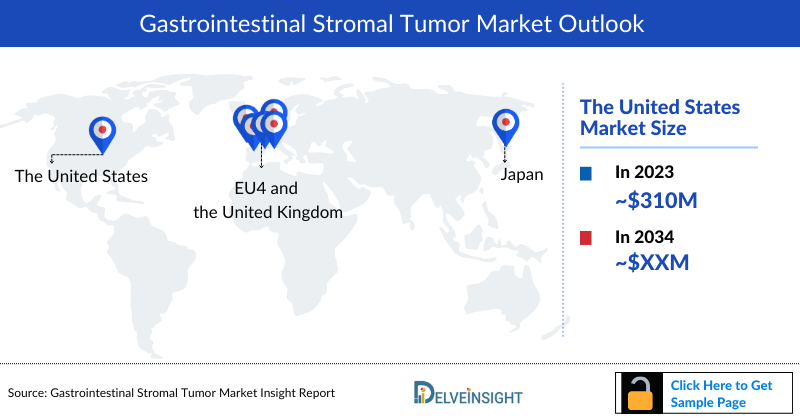
The total Gastrointestinal Stromal Tumor market size is analyzed for individual countries (the United States Market, EU4 (Germany, France, Italy, and Spain) and the UK market, and Japan). The United States accounted for a larger portion of the 7MM market for Gastrointestinal Stromal Tumor in 2023 due to the increasing prevalence of the condition and treatment demand. This dominance is predicted to continue with the potential early entry of new products.
Country-wise Market Size Distribution of Gastrointestinal Stromal Tumor
Gastrointestinal Stromal Tumor Market Size by Therapies
In the landscape of advanced GIST treatment, GLEEVEC (imatinib mesylate) stands as the approved first-line therapy, followed by SUTENT (sunitinib) as a second-line option for those resistant or intolerant to imatinib. In real-world scenarios, SUTENT or regorafenib may even be considered as first-line therapies in cases of intolerance or severe adverse events with imatinib. STIVARGA (regorafenib) and QINLOCK (ripretinib) are approved as third and fourth-line options, respectively. Notably, emerging therapies such as Crenolanib, Bezuclastinib, and others are being investigated for GIST and are anticipated to get approved during the forecast period (2024–2034).
The GIST Treatment Market Size Based on currently prescribed therapies as well as emerging therapies, in the US, is expected to grow by 2034. DelveInsight assessment of the emerging therapies indicates that the GIST Market will experience a major shift in the market with the launch of several therapies during the forecast period (2024-2034).
Key Findings
- The Gastrointestinal Stromal Tumor Therapeutics Market Size in the 7MM was around USD 450 million in 2023.
- The United States accounts for the highest Gastrointestinal Stromal Tumor Treatment Market Size approximately 69% of the total market size in 7MM in 2023, in comparison to the other major markets i.e., EU4 countries (Germany, Italy, France, and Spain) and the United Kingdom, and Japan.
- Among the EU4 and the UK, Germany had the highest Gastrointestinal Stromal Tumor Treatment Market size with USD 33 million in 2023, while Spain had the lest market size of GIST with about USD 17 million in 2023.
- The Gastrointestinal Stromal Tumor Treatment Market Size in Japan was estimated to be nearly USD 30 Million in 2023, which accounts for approximately 7% of the total 7MM market.
- With the expected launch of upcoming Gastrointestinal Stromal Tumor Therapies such as Crenolanib, Bezuclastinib (CGT9486/PLX9486), and others the total market of GIST is expected to show a decent change in upcoming years.
Gastrointestinal Stromal Tumor Drugs Uptake
This section focuses on the sales uptake of potential Gastrointestinal Stromal Tumor drugs that have recently launched or are anticipated to be launched in the Gastrointestinal Stromal Tumor market between 2020 and 2034. It estimates the market penetration of the Gastrointestinal Stromal Tumor drugs for a given country, examining their impact within and across classes and segments. It also touches upon the financial and regulatory decisions contributing to the drug’s probability of success (PoS) in the Gastrointestinal Stromal Tumor market. For example, for Crenolanib, we expect the drug uptake to be medium with a probability-adjusted peak share of around 7%, and years to the peak is expected to be 6 years from the year of launch in Japan.
Gastrointestinal Stromal Tumor Pipeline Development Activities
The report provides insights into Gastrointestinal Stromal Tumor clinical trials within Phase II and III stages. It also analyzes Gastrointestinal Stromal Tumor Companies involved in developing targeted therapeutics.
Pipeline Development Activities
The Gastrointestinal Stromal Tumor therapeutics market report covers information on collaborations, acquisition and merger, licensing, patent details, and other information for emerging Gastrointestinal Stromal Tumor therapies.
KOL Views
To keep up with current Gastrointestinal Stromal Tumor market trends and fill gaps in secondary findings, we interview KOLs and SMEs working in the Gastrointestinal Stromal Tumor domain. Their opinion helps understand and validate current and emerging therapies and treatment patterns or Gastrointestinal Stromal Tumor market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the Gastrointestinal Stromal Tumor unmet needs.
Gastrointestinal Stromal TumorTherapeutics Market: KOL Insights
DelveInsight’s analysts connected with 50+ KOLs to gather insights; however, interviews were conducted with 15+ KOLs in the 7MM. These KOLs were from organizations, institutes, and hospitals, such as New York University School of Medicine, New York, US; Oregon Health and Science University, Portland, OR, US; Medical Faculty Mannheim, University of Heidelberg, Mannheim, Germany; University of Rome “La Sapienza”, Rome, Italy; Cambridge University Hospitals, Cambridge, United Kingdom; Department of Clinical Oncology, Fujita Health University, Japan; and others. “In Italy, a large portion out of the overall population belongs to the age group of 45-65 years, which is also the reason behind the increase in cases of GIST.”
Gastrointestinal Stromal Tumor Therapeutics Market: Qualitative Analysis
We perform Qualitative and Gastrointestinal Stromal Tumor Therapeutics Market Intelligence analysis using various approaches, such as SWOT analysis, and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the Analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving Gastrointestinal Stromal Tumor Treatment Market Landscape.
Conjoint Analysis is done to analyze multiple approved and emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy. Further, the therapies’ safety is evaluated wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the route of administration, order of entry and designation, probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Gastrointestinal Stromal Tumor Market Access and Reimbursement
Reimbursement is a crucial point for any drug after its approval. Many drugs or therapies are not properly recognized by the reimbursement body and may fail to get reimbursed or their reimbursement process gets delayed.
DelveInsight’s ‘Gastrointestinal Stromal Tumor Treatment Market Insights, Epidemiology, and Market Forecast – 2034’ report provides a descriptive overview of the market access and reimbursement scenario of Gastrointestinal Stromal Tumor. This section includes a detailed analysis of the country-wise healthcare system for each therapy, enlightening the market access, reimbursement policies, and health technology assessments. The report further provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of approved therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Gastrointestinal Stromal Tumor Therapeutics Market Report Scope
- The Gastrointestinal Stromal Tumor Therapeutics Market Report covers a segment of key events, an executive summary, descriptive overview, explaining its causes, signs and symptoms, pathogenesis, and currently available therapies
- Comprehensive insight has been provided into the epidemiology segments and forecasts, the future growth potential of diagnosis rate, disease progression along with treatment guidelines
- Additionally, an all-inclusive account of both the current and emerging therapies along with the elaborative profiles of late-stage and prominent therapies will have an impact on the current Gastrointestinal Stromal Tumor Treatment Market Landscape
- A detailed review of the Gastrointestinal Stromal Tumor Therapeutics Market; historical and forecasted Gastrointestinal Stromal Tumor Treatment Market Size, Gastrointestinal Stromal Tumor Drugs Market Share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach
- The Gastrointestinal Stromal Tumor Treatment Market Report provides an edge while developing business strategies, by understanding trends, through SWOT analysis and expert insights/KOL views, patient journey, and treatment preference that help in shaping and driving the 7MM Gastrointestinal Stromal Tumor Drugs Market
Gastrointestinal Stromal Tumor Therapeutics Market Report Insights
- Gastrointestinal Stromal Tumor Patient Population
- Gastrointestinal Stromal Tumor Therapeutic Approaches
- Gastrointestinal Stromal Tumor Pipeline Drugs Analysis
- Gastrointestinal Stromal Tumor Treatment Market Size and Trends
- Existing and future Gastrointestinal Stromal Tumor Drugs Market Opportunity
Gastrointestinal Stromal Tumor Treatment Market Report Key Strengths
- 11 Years Gastrointestinal Stromal Tumor Market Forecast
- 7MM Coverage
- Gastrointestinal Stromal Tumor Epidemiology Segmentation
- Key Cross Competition
- Conjoint analysis
- Gastrointestinal Stromal Tumor Drugs Uptake
- Key GIST Market Forecast Assumptions
Gastrointestinal Stromal Tumor Treatment Market Report Assessment
- Current Gastrointestinal Stromal Tumor Treatment Market Practices
- Gastrointestinal Stromal Tumor Unmet Needs
- Gastrointestinal Stromal Tumor Pipeline Drugs Profiles
- Gastrointestinal Stromal Tumor Drugs Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
- Gastrointestinal Stromal Tumor Market Drivers
- Gastrointestinal Stromal Tumor Market Barriers
Key Questions Answered In The GIST Market Report
Gastrointestinal Stromal Tumor Treatment Market Insights:
- What was the Gastrointestinal Stromal Tumor Treatment Market Size, the Gastrointestinal Stromal Tumor Therapeutics Market Size by therapies, and market share (%) distribution in 2020, and how it would all look in 2034? What are the contributing factors for this growth?
- What are the unmet needs are associated with the current Gastrointestinal Stromal Tumor Treatment Market?
- How are emerging therapies going to contribute to the Gastrointestinal Stromal Tumor Market after approval?
- Which drug is going to be the largest contributor in 2034?
- What are the pricing variations among different geographies for approved and off-label therapies?
- How would the Gastrointestinal Stromal Tumor Market Drivers, Barriers, and future opportunities affect the Gastrointestinal Stromal Tumor Market dynamics and subsequent analysis of the associated trends?
Gastrointestinal Stromal Tumor Epidemiology Insights:
- What are the disease risk, burden, and Gastrointestinal Stromal Tumor Unmet Needs? What will be the growth opportunities across the 7MM concerning the patient population of Gastrointestinal Stromal Tumor?
- What is the historical and forecasted Gastrointestinal Stromal Tumor patient pool in the United States, EU4 (Germany, France, Italy, and Spain) the United Kingdom, and Japan?
- Why do only limited patients appear for diagnosis? Why is the current year diagnosis rate not high?
- What factors are affecting the diagnosis and treatment of the indication?
Current GIST Treatment Market Scenario, Marketed Drugs, and Emerging Therapies:
- What are the current options for the Gastrointestinal Stromal Tumor Treatment?
- What are the current treatment guidelines for the Gastrointestinal Stromal Tumor Treatment in the US and Europe?
- How many companies are developing therapies for the Gastrointestinal Stromal Tumor Treatment?
- How many emerging therapies are in the mid-stage and late stage of development for the treatment of Gastrointestinal Stromal Tumor?
- What are the recent novel therapies, targets, mechanisms of action, and technologies developed to overcome the limitation of existing therapies?
- What are the key designations that have been granted for the emerging therapies for Gastrointestinal Stromal Tumor?
- Patient acceptability in terms of preferred treatment options as per real-world scenarios?
- What are the country-specific accessibility issues of expensive, recently approved therapies? Focus on reimbursement policies.
- What are the 7MM historical and forecasted market of Gastrointestinal Stromal Tumor?
Reasons to buy Gastrointestinal Stromal Tumor Market Report
- The report will help in developing business strategies by understanding the latest trends and changing treatment dynamics driving the Gastrointestinal Stromal Tumor Market
- Insights on patient burden/disease prevalence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years
- To understand the existing Gastrointestinal Stromal Tumor Drugs Market opportunity in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
- Identification of strong upcoming players in the Gastrointestinal Stromal Tumor Drugs Market will help in devising strategies that will help in getting ahead of competitors.
- Detailed analysis and ranking of class-wise potential current and emerging therapies under the Conjoint analysis section to provide visibility around leading classes
- Highlights of Access and Reimbursement policies of approved therapies, barriers to accessibility of off-label expensive therapies, and patient assistance programs
- To understand the perspective of Key Opinion Leaders’ around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in future.
- Detailed insights on the unmet need of the existing Gastrointestinal Stromal Tumor Drugs Market so that the upcoming players can strengthen their development and launch strategy
Stay Updated with us for Recent Articles

-market.png&w=256&q=75)
-pipeline.png&w=256&q=75)


