Acute Myeloid Leukemia Market Summary
- The Acute Myeloid Leukemia Market is projected to grow at a significant CAGR by 2034 in the leading countries (the US, EU4, UK, and Japan).
- The leading Acute Myeloid Leukemia Companies such as Daiichi Sankyo, Agios Pharma, Astellas Pharma, Rigel Pharmaceuticals, Jazz Pharmaceuticals, Novartis, Pfizer, Abbvie, BMS, Arog Pharmaceuticals, Actinium Pharmaceutical, Astex Pharmaceutical, Syndax, Geron, SELLAS Life Sciences, Johnson & Johnson Innovative Medicine, Sanofi, Affimed, Sumitomo Pharma, Kura Oncology, CULLINAN THERAPEUTICS, Molecular Partners, Caribou Biosciences, BioPath Holdings, and others.
Acute Myeloid Leukemia Market and Epidemiology Analysis
- In 2023, the United States accounted for the largest Acute Myeloid Leukemia Treatment Market Size, in comparison to EU4 (Germany, France, Italy, and Spain) and the United Kingdom and Japan.
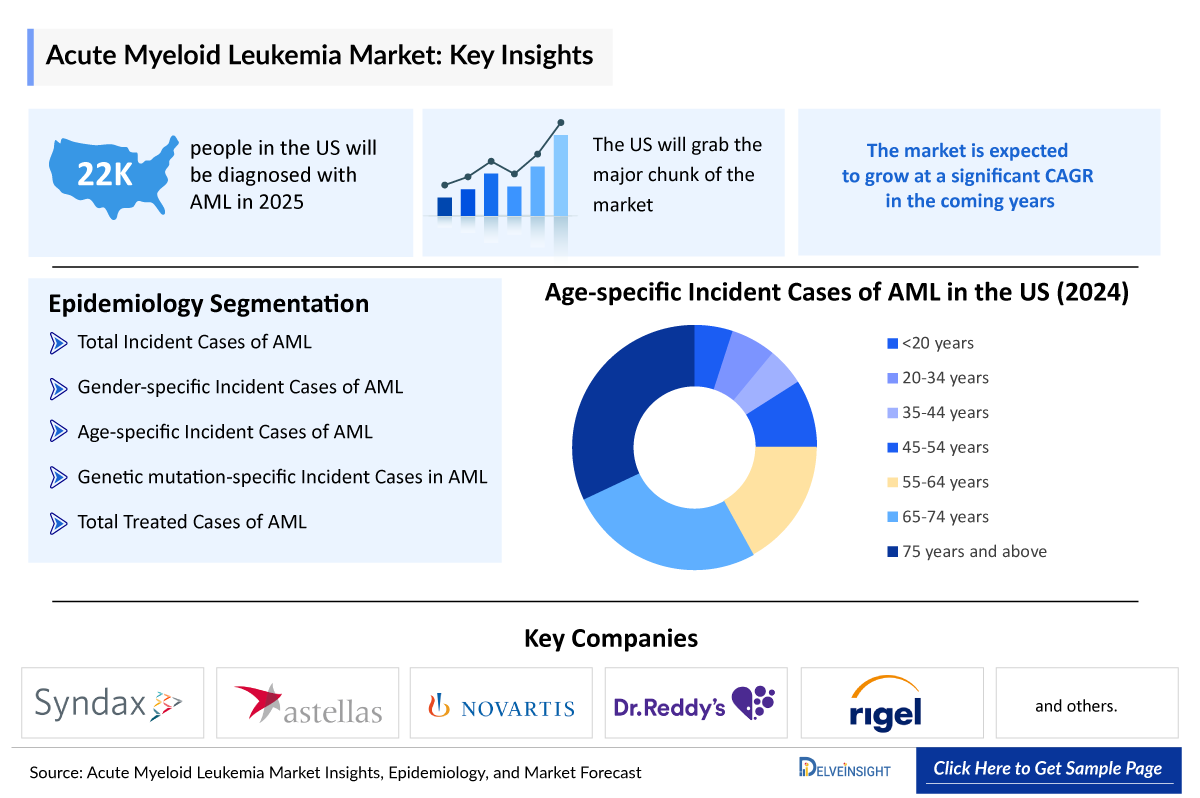
- The total 7MM Acute Myeloid Leukemia Incidence Cases in 2023 were 43,500, out of which the highest cases of this disease were seen in the United States.
- While the majority of newly Acute Myeloid Leukemia Diagnosed Patients receiving frontline therapy achieve remission, 30-40% will experience a relapse.
- The Acute Myeloid Leukemia Treatment Market Landscape has remained virtually unchanged for almost five decades. Approved Acute Myeloid Leukemia Drugs in frontline and relapse/refractory setting include VYXEOS (Jazz Pharmaceuticals), RYDAPT (Novartis), and ONUREG (BMS), DAURISMO (Pfizer), TIBSOVO (Agios Pharma), VENCLEXTA (Abbvie), IDHIFA (BMS), XOSPATA (Astellas Pharma), VANFLYTA (Daiichi Sankyo), REZLIDHIA (Rigel Pharmaceuticals), and others.
- Key Acute Myeloid Leukemia Companies including, Arog Pharmaceuticals (Crenolanib), Actinium Pharmaceutical (Iomab-B), Astex Pharmaceutical (ASTX030), Syndax (Revumenib), SELLAS Life Sciences (SLS009), Johnson & Johnson Innovative Medicine (JNJ-75276617), and others, are currently working on developing therapies for Acute Myeloid Leukemia.
- The KMT2A rearrangement is present in up to 15% of children and adults with acute leukemias and around 80% of infants with acute lymphoblastic leukemia. The NPM1 mutation is more common, occurring in up to 30% of Acute Myeloid Leukemia Patients.
- There are no approved targeted therapies for KMT2A disease. Menin inhibitors in clinical development have shown meaningful CR + CRh rates in KMT2A rearrangement or NPM1m acute leukemia populations (Syndax’s Revumenib 23% in KMT2A rearrangement, Kura’s Ziftomenib 35% in NPM1m, Janssen’s JNJ-75276617 21% in KMT2Ar/NPM1m populations).
- Revumenib is the sole drug to have Phase II data in KMT2A rearrangement AML, where the drug led to high remission rates with a predictable safety profile in pediatric and adult patients in this segment. Syndax expects an FDA decision on revumenib in KMT2Ar acute leukemia by December 26, 2024.
- As soon as Syndax receives regulatory clearance, it is ready for the launch of Revumenib. Prior to the much anticipated approval, Syndax is on target to complete plans that cover over 90% of all insured lives, including Part D and commercial.
- In February 2024, Gilead Sciences discontinued the Phase III ENHANCE-3 study and the US FDA placed all magrolimab studies in MDS and AML, including related expanded access programs, on full clinical hold. The discontinuation was issued based on the results of an interim analysis of this trial as well as the data from the ENHANCE and ENHANCE-2 studies that demonstrated futility and increased risk of death. With this the company will no longer pursue development of the treatment in hematologic cancers.
- Acute Myeloid Leukemia Market Growth is expected to be mainly driven by the entry of novel therapies with better clinical profiles, an increase in market penetration of targeted/advanced therapies, an upsurge in research and development, an enriched understanding of the disease, and increased Acute Myeloid Leukemia Incidence, and imminent drug launches. Whereas the high cost of therapy, toxic therapies, limitations in designing clinical trials, and low survival rates halt the market growth.
Request for Unlocking the Sample Page of the "Acute Myeloid Leukemia Treatment Market"
Key Factors Driving the Acute Myeloid Leukemia Market Growth
-
Increasing Incidence of AML
The global prevalence of AML is rising, particularly among older populations, leading to a higher demand for effective treatments.
-
Advancements in Research and Technology
Ongoing research and technological innovations are enhancing the understanding of AML, facilitating the development of targeted therapies and improving treatment outcomes.
-
Early Diagnosis and Screening Programs
Efforts to implement early diagnosis and screening programs are leading to the detection of AML at earlier stages, improving prognosis and treatment efficacy.
-
Improved Healthcare Infrastructure
Enhancements in healthcare infrastructure are facilitating better diagnosis and treatment of AML, particularly in emerging markets.
DelveInsight’s "Acute Myeloid Leukemia Treatment Market Insight, Epidemiology, and Market Forecast – 2034" report delivers an in-depth understanding of Acute Myeloid Leukemia, historical and forecasted epidemiology as well as the Acute Myeloid Leukemia market trends in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
The Acute Myeloid Leukemia Treatment Market Report provides current treatment practices, emerging drugs, Acute Myeloid Leukemia market share of individual therapies, and current and forecasted Acute Myeloid Leukemia market size from 2020 to 2034, segmented by seven major markets. The report also covers current Acute Myeloid Leukemia treatment market practices/algorithms and unmet medical needs to curate the best of the opportunities and assess the underlying potential of the market.
Scope of the Acute Myeloid Leukemia Market | |
|
Study Period |
2020–2034 |
|
Forecast Period |
2024–2034 |
|
Geographies Covered |
|
|
Acute Myeloid Leukemia Epidemiology |
Segmented by:
|
|
Acute Myeloid Leukemia Companies |
Daiichi Sankyo, Agios Pharma, Astellas Pharma, Rigel Pharmaceuticals, Jazz Pharmaceuticals, Novartis, Pfizer, Abbvie, BMS, Arog Pharmaceuticals, Actinium Pharmaceutical, Astex Pharmaceutical, Syndax, Geron, SELLAS Life Sciences, Johnson & Johnson Innovative Medicine, Sanofi, Affimed, Sumitomo Pharma, Kura Oncology, CULLINAN THERAPEUTICS, Molecular Partners, Caribou Biosciences, BioPath Holdings, and others |
|
Acute Myeloid Leukemia Drugs |
VYXEOS, ONUREG, REZLIDHIA, TIBSOVO, IDHIFA, VANFLYTA, RYDAPT, DAURISMO, VENCLEXTA, XOSPATA, Crenolanib, ASTX030, Revumenib, ziftomenib, Imetelstat, AFM28, MP0533, SAR443579, BP1001, JNJ-75276617, DSP-5336, CB-012, CLN-049 and others |
|
Acute Myeloid Leukemia Drugs Market |
Segmented by:
|
|
Analysis |
|
Acute Myeloid Leukemia Treatment Market: Understanding and Algorithm
Acute myeloid leukemia is a heterogeneous hematologic malignancy characterized by the clonal expansion of myeloid blasts in the peripheral blood, bone marrow, and/or other tissues. AML can involve tissues outside the bone marrow and blood, including lymph nodes, the brain, skin, and other body parts. The classification of AML depends on the etiology, genetics, immunophenotype, and morphology. Myelodysplastic syndrome is by far the most typical risk factor for AML. Myelofibrosis and aplastic anemia are two other hematological conditions increasing the risk of AML. The main Acute Myeloid Leukemia symptoms are caused by a lack of normal blood cells; as AML develops quickly, people usually report feeling unwell for only a short period (days or weeks) before they are diagnosed.
Acute Myeloid Leukemia Diagnosis
For many years the Acute Myeloid Leukemia diagnosis was based solely on pathologic and cytological examination of bone marrow and peripheral blood smears. Initially proposed in 1976, the FAB established a classification method that divides the AML into eight different subtypes according to the morphological appearance of blasts and their reactivity with histochemical stains. A way to recognize and classify different subgroups of AML through clinical, morphological, and genetic correlation was proposed by the WHO, which made a new classification that was updated in 2008. This classification has important differences from the classification of the FAB. Over the time, Various tests and examinations are involved in the accurate diagnosis and Acute Myeloid Leukemia classification into different subtypes, including, complete blood count (CBC), flow cytometry, bone marrow aspiration and biopsy, cytogenetic analysis, molecular testing, and immunophenotyping. Various imaging tests such as PET/CT scan or CT-guided needle biopsy are also required to make accurate diagnosis.
Further details related to diagnosis will be provided in the report…
Acute Myeloid Leukemia Treatment
Treatment of most patients with AML typically consists of two chemotherapy (chemo) phases:
- Remission induction (often just called induction)
- Consolidation (post-remission therapy)
Remission is the treatment target, and the current standard of care for AML relies on intense chemotherapy-based induction, consolidation therapy, and hematopoietic stem cell transplantation. Intensive therapy, which can be physically and mentally taxing, is not appropriate for all patients and is usually utilized with healthier, younger individuals. Despite induction therapy, there is still minimal residual disease for which consolidation therapy is initiated to prevent any relapse risk by eliminating the residual disease. Stem cell transplants are intensive treatments with real risks of serious complications, including death, and their exact role in treating AML is not always clear.
If further treatment or a clinical trial is not an option, the focus of treatment may shift to controlling symptoms caused by leukemia rather than trying to cure it. This is called palliative treatment or supportive care. Treatments that may be helpful in such cases include radiation therapy and appropriate pain-relieving medicines. If medicines such as aspirin and ibuprofen do not help with the pain, stronger opioid medicines such as morphine are likely helpful.
Acute Myeloid Leukemia Epidemiology
The acute myeloid leukemia epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by the total Acute Myeloid Leukemia Incidence cases, Acute Myeloid Leukemia Gender-specific cases, Acute Myeloid Leukemia age-specific cases, and Acute Myeloid Leukemia genetic mutations in the 7MM covering the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan from 2020 to 2034.
Acute Myeloid Leukemia Epidemiological Analysis and Forecast
- In the 7MM, the US accounted for the highest number of Acute Myeloid Leukemia Incidence Cases, i.e., approximately 21,300 cases in 2023.
- Among EU4 and the UK, Germany accounted for the highest number of cases of Acute Myeloid Leukemia, whereas Spain occupied the bottom of the ladder.
- As per DelveInsight’s estimates, it has been observed that Acute Myeloid Leukemia is slightly more common in males (~52%) than in females (~48%).
Acute Myeloid Leukemia Epidemiology Segmentation
- Total Acute Myeloid Leukemia Incidence cases
- Acute Myeloid Leukemia Gender-specific cases
- Acute Myeloid Leukemia Age-specific cases
- Acute Myeloid Leukemia Genetic Mutations
- Acute Myeloid Leukemia Line-wise Treated Pool
- AML can occur in children, but it is uncommon in people under the age of 45. The average age of people when they are first diagnosed with AML is about 69.
- Among all the genetic mutations in AML, NPM1 gene mutation accounted for the majority of the cases.
Acute Myeloid Leukemia Market Recent Developments and Breakthroughs
- In September 2025, Akeso Inc. (9926.HK) announced that its monoclonal antibody ligufalimab (AK117) received FDA Orphan Drug Designation for treating acute myeloid leukemia (AML). This status offers development support, tax benefits, and up to seven years of market exclusivity upon approval.
- In July 2025, ImCheck Therapeutics announced that the FDA granted Orphan Drug Designation to ICT01, their humanized anti-BTN3A monoclonal antibody targeting γ9δ2 T cells, for treating acute myeloid leukemia (AML). AML is especially challenging to treat in older or unfit patients ineligible for intensive chemotherapy.
- In July 2025, the FDA accepted the supplemental new drug application for INQOVI (decitabine and cedazuridine) plus venetoclax to treat adults with newly diagnosed acute myeloid leukemia (AML) who cannot undergo intensive chemotherapy. The FDA set a standard review with a decision expected by February 25, 2026. This is based on Phase 2b study results from ASCERTAIN-V.
- In July 2025, Syndax Pharmaceuticals announced that the FDA granted Priority Review for its supplemental New Drug Application (sNDA) for Revuforj (revumenib), targeting relapsed or refractory mutant NPM1 acute myeloid leukemia (AML). The FDA’s Real-Time Oncology Review program is expediting the process, with a PDUFA action date set for October 25. Revuforj is an oral menin inhibitor initially approved in 2024 for acute leukemia with KMT2A translocation.
- In June 2025, Senti Biosciences announced FDA orphan drug designation for SENTI-202 to treat relapsed/refractory hematologic malignancies, including AML.
- In June 2025, Kura Oncology and Kyowa Kirin announced the FDA has accepted Kura’s NDA for ziftomenib to treat adult relapsed or refractory AML with NPM1 mutation. The application received Priority Review, with a PDUFA date of November 30, 2025.
- In June 2025, Moleculin Biotech received FDA agreement on a single pediatric approval study to evaluate Annamycin combined with Cytarabine as a second-line treatment for pediatric relapsed/refractory acute myeloid leukemia (R/R AML).
- In March 2025, CERo Therapeutics Holdings, Inc. announced that the FDA gave a positive review on an amendment to its IND regarding Chemistry, Manufacturing, and Controls (CMC). This completes the company's final commitment to the FDA before initiating patient dosing and shortens the manufacturing timeline by about a week.
- In February 2025, Moleculin Biotech, Inc.announced that it received FDA feedback and guidance on its IND amendment, allowing a reduction in the size of its Phase 3 pivotal trial protocol. The trial is evaluating Annamycin in combination with Cytarabine (AnnAraC) for the treatment of AML patients who are refractory to or relapsed after induction therapy (R/R AML) (MB-108).
- In February 2025, Kura Oncology and Kyowa Kirin announced positive topline results from KOMET-001, a Phase 2 registration-directed trial of ziftomenib, a once-daily oral menin inhibitor, in patients with relapsed/refractory NPM1-mutant acute myeloid leukemia (AML).
- In February 2025, Auron Therapeutics, a clinical-stage biotechnology company focused on cell-state plasticity to improve outcomes in oncology and inflammatory diseases, announced progress in its lead KAT2A/B program and clinical candidate, AUTX-703. The company also completed a $27 million Series B financing.
- In Jan 2025, Medexus announced that the FDA approved GRAFAPEX™, an alkylating agent, with fludarabine as a preparative regimen for allogeneic hematopoietic stem cell transplantation (alloHSCT) in adult and pediatric patients aged one year and older with acute myeloid leukemia (AML) or myelodysplastic syndrome (MDS).
- In Jan 2025, PureTech Health announced that the FDA granted Fast Track designation to its investigational drug LYT-200 for treating acute myeloid leukemia (AML). This designation aims to accelerate the drug’s development and review process due to the critical need for new AML treatments.
- In Nov 2024, Shorla Oncology, a U.S.-Ireland specialty pharmaceutical company, announced that the FDA approved IMKELDI (imatinib) oral solution, the first oral liquid form of imatinib for treating certain forms of leukemia and other cancers.
- In Nov 2024, Syndax Pharmaceuticals announced that the FDA approved Revuforj® (revumenib) as the first and only menin inhibitor for treating relapsed or refractory (R/R) acute leukemia with a lysine methyltransferase 2A gene (KMT2A) translocation in adult and pediatric patients aged one year and older.
- In Nov 2024, Lin BioScience (6696, Taiwan OTC) announced that its lead pipeline, LBS-007, received Fast Track Designation from the FDA for the treatment of acute myeloid leukemia.
- In October 2024, the FDA granted fast-track designation to the novel GCN2 kinase modulator HC-7366 for treating adult patients with relapsed or refractory acute myeloid leukemia (AML).
- In September 2024, ImCheck Therapeutics announced that the FDA granted Fast Track designation to ICT01 in combination with azacitidine and venetoclax for treating acute myeloid leukemia (AML) patients aged 75 or older, or those with comorbidities that prevent standard chemotherapy. This decision follows encouraging results from the Phase 1 EVICTION study.
Acute Myeloid Leukemia Drugs Analysis
Acute Myeloid Leukemia Marketed Drugs
-
VANFLYTA (quizartinib): Daiichi Sankyo
Quizartinib is an oral, highly potent type II FLT3 inhibitor that selectively targets FLT3-ITD mutations and has been specifically developed for patients with FLT3-ITD positive AML.
In July 2023, the US FDA approved VANFLYTA (quizartinib), with standard cytarabine and anthracycline induction and cytarabine consolidation, and as maintenance monotherapy following consolidation chemotherapy, for the treatment of adult patients with newly Acute Myeloid Leukemia diagnosed that is FLT3 internal tandem duplication (ITD)-positive. Later in September, the drug got approval in Europe for the same patient segment. Quizartinib also is approved in Japan for FLT3-ITD mutation positive AML, and as a monotherapy for relapsed/refractory AML that is FLT3-ITD positive. The quizartinib clinical development program includes a Phase I/II trial in pediatric and young adult patients with relapsed/refractory FLT3-ITD positive AML in Europe and North America and several Phase I/II combination studies as part of a strategic collaboration with the University of Texas MD Anderson Cancer Center.
-
ONUREG (azacitidine): Celgene/Bristol Myers Squibb
ONUREG is a nucleoside metabolic inhibitor, and its main Acute Myeloid Leukemia mechanism of action is the hypomethylation of DNA and direct cytotoxicity to abnormal hematopoietic cells in the bone marrow.
The drug received FDA approval in September 2020, for continued treatment of adult patients with AML who achieved first complete remission (CR) or complete remission with incomplete blood count recovery (CRi) following intensive induction chemotherapy and cannot complete intensive curative therapy. It is the first oral azacitidine product and FDA-approved therapy for continued AML therapy for patients in remission. Later in June 2021, the drug got approved in Europe as well as frontline oral maintenance therapy for adults with AML.
Comparison of Marketed Therapies | ||||
|
Drugs |
Company Name |
MoA |
Indication |
Approval (US) |
|
VANFLYTA |
Daiichi Sankyo |
FLT3 inhibitor |
FLT3-ITD mutation positive newly diagnosed AML |
2023 |
|
ONUREG |
Celgene/Bristol Myers Squibb |
DNA methylation inhibitors |
AML patients who achieved first complete remission |
2020 |
|
TIBSOVO |
Servier Laboratories |
IDH1 inhibitor |
Newly diagnosed AML and R/R AML |
2018 (R/R AML with an IDH1 Mutation); 2019 (newly diagnosed AML with an IDH1 Mutation); 2022 (in combination with azacitidine newly diagnosed AML with an IDH1 Mutation) |
Acute Myeloid Leukemia Emerging Drugs
-
Crenolanib: Arog Pharmaceuticals
Crenolanib is a small molecule investigational drug candidate developed by Arog Pharmaceuticals. It is a potent small molecule inhibitor of wild-type and mutant forms of FLT3 (FMS-like Tyrosine Kinase 3) and PDGFRα/β (Platelet-Derived Growth Factor Receptor). The drug has been granted ODD and FTD by the US FDA. Crenolanib combined with chemotherapy is currently being studied in two Phase III clinical trials for patients with newly diagnosed AML (NCT03258931) and R/R AML (NCT03250338). Results from a completed Phase II study in newly diagnosed acute myeloid leukemia patients with FLT3 mutations were presented at the ASCO 2024 annual meeting, where crenolanib plus intensive chemotherapy in adults with newly diagnosed FLT3-mutant AML resulted in high rate of deep responses and long-term survival with acceptable toxicity.
-
SLS009: SELLAS Life Sciences
SLS009 is a potential first- and best-in-class, highly selective, differentiated small molecule CDK9 inhibitor. Compared with other CDK9 inhibitors, SLS009 has reduced toxicity and increased potency. In patients with AML, data has shown SLS009 to demonstrate a high response rate, specifically among those with unfavorable prognostic factors like an ASXL1 mutation. Recently, in July 2024, the FDA granted SLS009 a rare pediatric disease designation (RPDD) for the treatment of pediatric patients AML. Previously, the drug has also been granted an orphan drug designation for the treatment of relapsed/refractory AML, and in January 2024, a fast track designation was granted by the FDA to SLS009 for this same indication.
SLS009 is currently being evaluated in a Phase I/II trial in Patients with Hematologic Malignancies including relapsed/refractory AML. In March 2024, the company reported positive topline findings from the Phase IIa portion of the Phase I/II where SLS009 combined with azacitidine and venetoclax showed that overall response rate (ORR) was 50% among patients with relapsed/refractory AML treated with the agent at the selected optimal dose of 30 mg twice weekly, exceeding the targeted 20% rate.
Comparison of Emerging Drugs | ||||
|
Product |
Company |
Phase |
Indications |
MoA |
|
Crenolanib |
Arog Pharmaceuticals |
III |
Newly diagnosed AML and R/R AML |
FLT3 inhibitor |
|
Iomab-B |
Actinium Pharmaceutical |
III |
R/R AML |
Ionizing radiation emitters |
|
Azacitidine and cedazuridine (ASTX030) |
Astex Pharmaceutical |
II/III |
AML |
DNMT inhibitor and CDA inhibitors |
|
Revumenib |
Syndax |
II |
R/R KMT2Ar acute leukemia |
MENIN-KMT2A inhibitor |
|
SLS009 |
SELLAS Life Sciences |
I/II |
R/R AML |
CDK9 inhibitor |
2024 Conferences Key Highlights
|
Drug |
Company |
Title |
Abstract |
Result |
|
European Hematology Association (EHA) 2024 | ||||
|
JNJ-75276617 |
A Phase Ib study of the MENIN-KMT2A inhibitor JNJ-75276617 in combination with venetoclax and azacitidine in relapsed/refractory acute myeloid leukemia with alterations in KMT2A or NPM1 |
S113 |
| |
|
Revumenib |
Revumenib monotherapy in patients with relapsed/refractory KMT2Ar acute leukemia: Topline efficacy and safety results from the pivotal AUGMENT-101 Phase II study |
S131 |
| |
|
Revumenib + azacitidine + venetoclax |
Phase Ib study of azacitidine, venetoclax and revumenib in newly diagnosed older adults with NPM1 mutated or KMT2A rearranged AML: Interim results of dose escalation from the BEAT AML consortium |
S134 |
| |
|
DSP-5336 |
First-in-human Phase I/II study of the MENIN-MLL inhibitor DSP-5336 in patients with relapsed or refractory acute leukemia: Updated results from dose escalation |
S132 |
| |
|
Magrolimab |
Magrolimab vs. placebo in combination with venetoclax and azacitidine in previously untreated patients with acute myeloid leukemia who are ineligible for intensive chemotherapy: The ENHANCE-3 study |
S138 |
| |
|
SAR443579 |
Completed dose escalation from the first-in-human, Phase I/II study of CD123 NK cell engager, SAR443579, in relapsed or refractory acute myeloid leukemia or high risk-myelodysplasia |
S146 |
| |
|
ASCO 2024 | ||||
|
BP1001 |
Interim safety and efficacy of BP1001 in a Phase II acute myeloid leukemia (AML) study. |
6511 |
| |
Acute Myeloid Leukemia Drugs Market Insights
FLT3 inhibitors: In some people with AML, the leukemia cells have a change (mutation) in the FLT3 gene. Drugs called FLT3 inhibitors target AML cells with this gene change. FLT3 inhibitors such as midostaurin (RYDAPT), quizartinib (VANFLYTA), and gilteritinib (XOSPATA) are now approved to treat people whose AML cells have an FLT3 mutation. Two generations of FLT3 inhibitors have been developed, each with varying levels of potency and specificity. Research has shown that these inhibitors enhance BCL-2 dependence while reducing BCL-xL and MCL-1 expression. This combination synergistically triggers apoptosis and increases the sensitivity of FLT3-mutated AML cells to venetoclax.
IDH inhibitors: IDH inhibitors offer promising treatment options for patients with relapsed or refractory AML who have IDH mutations. However, for those newly diagnosed with IDH-mutated AML, these inhibitors may not be the most effective due to their low complete remission rates. While the safety of IDH inhibitors is generally manageable, physicians must remain vigilant in monitoring and addressing the differentiation syndrome adverse events associated with their use. Some of these drugs, such as IDHIFA, REZLIDHIA, and TIBSOVO, are now approved to treat AML with certain IDH gene mutations. Several other IDH inhibitors are now being studied as well.
BCL-2 inhibitors: Some people with AML have leukemia cells that make too much of a protein called BCL-2. Leukemia cells that have too much BCL-2 tend to be harder to kill with chemo drugs. BCL-2 inhibitors prevent the BCL-2 protein from working in cancer cells. VENCLEXTA is a BCL-2 inhibitor that has been approved to treat AML with too much BCL-2 protein. Several other BCL-2 inhibitors are being studied as well.
Acute Myeloid Leukemia Market Outlook
The goal of Acute Myeloid Leukemia treatment is remission and current conventional therapeutic options for AML rely on intensive chemotherapy-based induction and consolidation therapy, together with hematopoietic stem cell transplantation. However, not all patients are eligible for this intensive therapy, which can be both physically and mentally demanding, and this approach is more frequently used in healthy, younger patients.
The standard Acute Myeloid Leukemia treatment involves cytarabine combined with an anthracycline (e.g., daunorubicin). This regimen has been the backbone of Acute Myeloid Leukemia treatment for decades, particularly for younger, fit patients. Allogeneic stem cell transplantation remains a potentially curative option for AML, particularly for patients in remission. There exist a number of established targeted therapies for treating AML like RYDAPT, XOSPATA and others target FLT3 mutations, which are present in approximately 30% of AML patients. TIBSOVO and IDHIFA are used to treat AML patients with IDH1 and IDH2 mutations, respectively. VENCLEXTA, a BCL-2 Inhibitor, is used in combination with hypomethylating agents or low-dose cytarabine for patients who are not candidates for intensive chemotherapy, and many others.
The Acute Myeloid Leukemia Drugs Market is seeing a surge in novel therapies, including targeted therapies, immunotherapies, and cell-based therapies. Ongoing research is exploring the use of monoclonal antibodies, CAR-T cell therapy, and checkpoint inhibitors in AML. The market for AML treatments is projected to grow significantly over the next few years with pharmaceutical Acute Myeloid Leukemia Companies like Arog Pharmaceuticals, Actinium Pharmaceutical, Astex Pharmaceutical, Syndax, SELLAS Life Sciences, Johnson & Johnson Innovative Medicine, and others actively developing and marketing AML therapies.
- Among the 7MM, the US accounted for the largest Acute Myeloid Leukemia Treatment Market Size.
- Among EU4 and the UK, Germany accounted for the highest Acute Myeloid Leukemia Treatment Market Size in 2023, while Spain occupied the lowest.
- There is currently no approved targeted therapy for the NPM1-mutant AML subgroup, resulting in high unmet need. NPM1-mutant AML represents approximately 30% of new AML cases annually. Ziftomenib became the first investigational treatment to be granted breakthrough therapy designation in NPM1-mutant AML in April 2024.
- Syndax has a strong clinical development plan set up for Revumenib, which will enable its use in acute leukemias with mutant NPM1 and KMT2A rearrangements across the treatment paradigm. The current clinical development plan of Revumenib extends beyond the initial relapsed or refractory indications. With Revumenib’s ongoing trials in combination with standards of care (SoC) may potentially support and extend the use in the frontline relapse, refractory and post-transplant maintenance settings.
Further details will be provided in the report….
Acute Myeloid Leukemia Pipeline Development Activities
The Acute Myeloid Leukemia therapeutics market report provides insights into different therapeutic candidates in Phase III, Phase II, and Phase I/II stage. It also analyzes key Acute Myeloid Leukemia Companies involved in developing targeted therapeutics. The Acute Myeloid Leukemia therapeutics market report covers detailed information on collaborations, acquisitions and mergers, licensing, and patent details for Acute Myeloid Leukemia emerging therapies.
Latest KOL- Views on Acute Myeloid Leukemia
To keep up with current market trends, we take KOLs and SMEs' opinions working in the domain through primary research to fill the data gaps and validate our secondary research. Some of the leaders like MD, Professor and Vice Chair Department of Critical Care Medicine and Director, PhD, and others. Their opinion helps to understand and validate current and emerging therapies and treatment patterns or Acute Myeloid Leukemia market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
Delveinsight’s analysts connected with 20+ KOLs to gather insights; however, interviews were conducted with 10+ KOLs in the 7MM. Centers such as the Johns Hopkins University School of Medicine, University of California, etc., were contacted. Their opinion helps understand and validate AML epidemiology and market trends.
Acute Myeloid Leukemia KOL Views |
|
“Acute myeloid leukemia treatment is a promising story since over the past ten years, patients have benefited from numerous innovative medicines and a better understanding of the biology of the illness. Nevertheless, there is still a significant medical need for it because the fatality rate is too high. Patients with favorable-risk acute myeloid leukemia had 5-year overall survival rates of 70 – 80%. Certainly, we want the overall survival to be much higher than that.” |
|
“Future improvements in the management of leukemia and lymphoma will result from a careful review of current management strategies. We are fortunate to have access to a variety of effective alternatives, and we are currently comparing side effects and quality of life to see which will keep our patients the safest and maintain their responses for the longest. We have several options. If the patient is in good health, you might consider using FLT3 inhibitors like midostaurin or antibodies like gemtuzumab ozogamicin in addition to severe chemotherapy. Hypomethylating medications combined with venetoclax (Venclexta) or the recently approved ivosidenib may be an alternative if the patient is unable to receive chemotherapy.” |
Acute Myeloid Leukemia Therapeutics Market: Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT and conjoint analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the Analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving treatment landscape.
The analyst analyzes multiple emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. In efficacy, the trial’s primary and secondary outcome measures are evaluated. Further, the therapies’ safety is evaluated wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials.
Acute Myeloid Leukemia Therapeutics Market Access and Reimbursement
PANO stands for Patient Assistance Now Oncology is a patient support program provided by the Novartis for RYDAPT, to help the patient get started with the medicine and provide information and resources throughout the treatment.The PANO SRF is a single form with two parts: 1) The patient and 2)The health care professional (HCP). Both parts must be fully completed and submitted to open a case. No follow-up is necessary. PANO will match the two parts and contact the HCP once both parts are received or if the information submitted is incomplete.
The Genentech Oncology Co-pay Assistance Program helps people with commercial health insurance. This might be a plan patients get through the employer or one purchased through a Health Insurance Marketplace. If the patient has commercial health insurance and meets other eligibility criteria, the Genentech Oncology Co-pay Assistance Program for VENCLEXTA may be able to help in paying for medicine. With this program, the patient may pay as little as USD 5 per prescription for VENCLEXTA + azacitidine, or VENCLEXTA + decitabine, or VENCLEXTA + low-dose cytarabine, up to a USD 25,000 yearly limit.
Revumenib Market Access
Considering that the PDUFA action date for the Revumenib is December 26, 2024, regulatory clearance is expected soon. In terms of market access, Syndax has put together a highly skilled team with a considerable amount of expertise collaborating with payers and other trade partners to streamline the introduction of Revumenib. Syndax's payers field team, in collaboration with the Medical Affairs team, is actively pursuing pre-approval information exchange with payers. The company aims to get plans that cover over 90% of all covered lives, including commercial and Part D, before the anticipated approval. Payers are aware about the unmet need in KMT2A-rearranged acute leukemias. The Company anticipates that, after approval, payers will decide on their formulary within six to twelve months. Additionally, Syndax anticipates that plans will allow patients to obtain the medicine through the medical exception process at launch, given the urgent need of the patients. Leading, best-in-class specialty pharmacies that are well-known for their capacity to assist clinicians and patients in gaining access to cutting-edge cancer medications have partnered with Syndax.
Further detailed analysis will be provided in the report….
Acute Myeloid Leukemia Therapeutics Market Report Scope
- The Acute Myeloid Leukemia therapeutics market report covers a descriptive overview, explaining its causes, signs and symptoms, pathogenesis, and currently available therapies.
- Comprehensive insight has been provided into Acute Myeloid Leukemia epidemiology and treatment.
- Additionally, an all-inclusive account of both the current and emerging therapies for Acute Myeloid Leukemia is provided, along with the assessment of new therapies, which will have an impact on the current Acute Myeloid Leukemia Treatment Market Landscape.
- A detailed review of the Acute Myeloid Leukemia Treatment Market; historical and forecasted is included in the report, covering the 7MM drug outreach.
- The Acute Myeloid Leukemia Treatment Market Report provides an edge while developing business strategies, by understanding trends shaping and driving the 7MM Acute Myeloid Leukemia drugs market.
Acute Myeloid Leukemia Treatment Market Report Insights
- Patient-based Acute Myeloid Leukemia Market Forecasting
- Therapeutic Approaches
- Acute Myeloid Leukemia Pipeline Drugs Analysis
- Acute Myeloid Leukemia Market Size and Trends
- Acute Myeloid Leukemia Drugs Market Opportunities
- Impact of Upcoming Acute Myeloid Leukemia Therapies
Acute Myeloid Leukemia Therapeutics Market Report Key Strengths
- 11 Years Acute Myeloid Leukemia Market Forecast
- 7MM Coverage
- Acute Myeloid Leukemia Epidemiology Segmentation
- Key Cross Competition
- Highly Analyzed Acute Myeloid Leukemia Drugs Market
- Acute Myeloid Leukemia Drugs Uptake
Acute Myeloid Leukemia Therapeutics Market Report Assessment
- Current Acute Myeloid Leukemia Treatment Market Practices
- Acute Myeloid Leukemia Unmet Needs
- Acute Myeloid Leukemia Pipeline Drugs Analysis Profiles
- Acute Myeloid Leukemia Drugs Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
Key Questions Answered in the Acute Myeloid Leukemia Market Report
Acute Myeloid Leukemia Market Insights
- What was the Acute Myeloid Leukemia drugs market share (%) distribution in 2020 and what it would look like in 2034?
- What would be the Acute Myeloid Leukemia treatment market size as well as market size by therapies across the 7MM during the study period (2020–2034)?
- What are the key findings about the Acute Myeloid Leukemia drugs market across the 7MM and which country will have the largest Acute Myeloid Leukemia market size during the study period (2020–2034)?
- At what CAGR, the Acute Myeloid Leukemia drugs market is expected to grow at the 7MM level during the study period (2020–2034)?
- What would be the Acute Myeloid Leukemia market growth till 2034 and what will be the resultant market size in the year 2034?
- What are the disease risks, burdens, and Acute Myeloid Leukemia unmet needs?
- What is the historical Acute Myeloid Leukemia patient pool in the United States, EU4 (Germany, France, Italy, and Spain), and the UK, and Japan?
- What will be the growth opportunities across the 7MM concerning the patient population of Acute Myeloid Leukemia?
- At what CAGR the population is expected to grow across the 7MM during the study period (2020–2034)?
- How many emerging therapies are in the mid-stage and late stage of development for the treatment of Acute Myeloid Leukemia?
- What are the key collaborations (Industry–Industry, Industry-Academia), Mergers and acquisitions, and licensing activities related to Acute Myeloid Leukemia therapies?
- What are the recent novel therapies, targets, mechanisms of action, and technologies developed to overcome the limitations of existing therapies?
- What are the Acute Myeloid Leukemia clinical trials going on and their status?
- What are the key designations that have been granted for the Acute Myeloid Leukemia emerging therapies?
Reasons to Buy the Acute Myeloid Leukemia Market Report
- The Acute Myeloid Leukemia therapeutics market report will help in developing business strategies by understanding trends shaping and driving Acute Myeloid Leukemia.
- To understand the future market competition in the Acute Myeloid Leukemia drugs market and Insightful review of the SWOT analysis of Acute Myeloid Leukemia.
- Organize sales and marketing efforts by identifying the best opportunities for Acute Myeloid Leukemia in the US, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
- Identification of strong upcoming players in the Acute Myeloid Leukemia drugs market will help in devising strategies that will help in getting ahead of competitors.
- Organize sales and marketing efforts by identifying the best opportunities for the Acute Myeloid Leukemia drugs market.
- To understand the future market competition in the Acute Myeloid Leukemia Drugs Market.
Stay updated with us for Recent Articles