Hereditary Angioedema Market
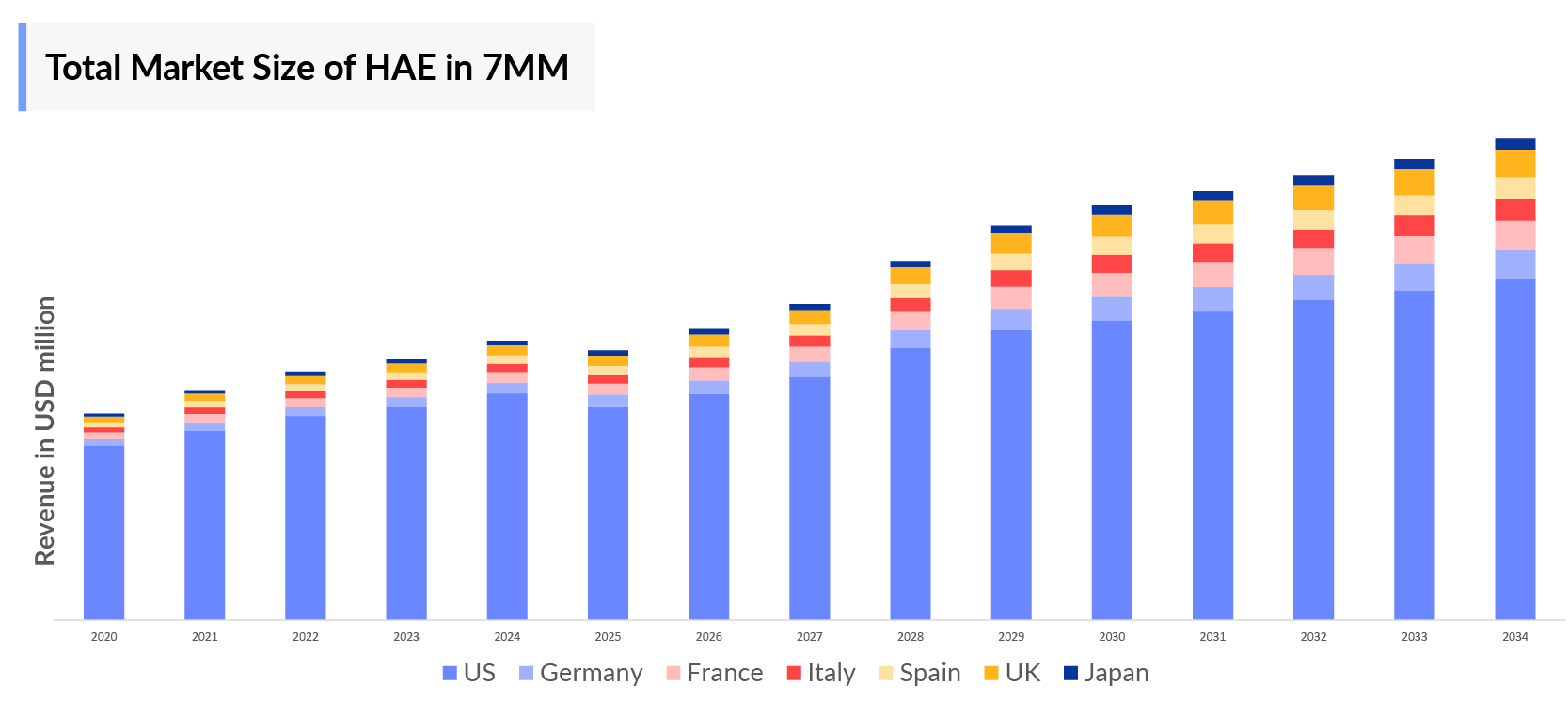
- The total Hereditary angioedema market size was found to be ~USD 3,000 million in 2023, which with the approval of many new therapies in the Hereditary angioedema market is further expected to increase in the forecasted period.
- Hereditary Angioedema is a rare genetic disorder characterized by recurrent episodes of severe swelling (angioedema) that can affect various parts of the body, including the limbs, face, gastrointestinal tract, and airway.
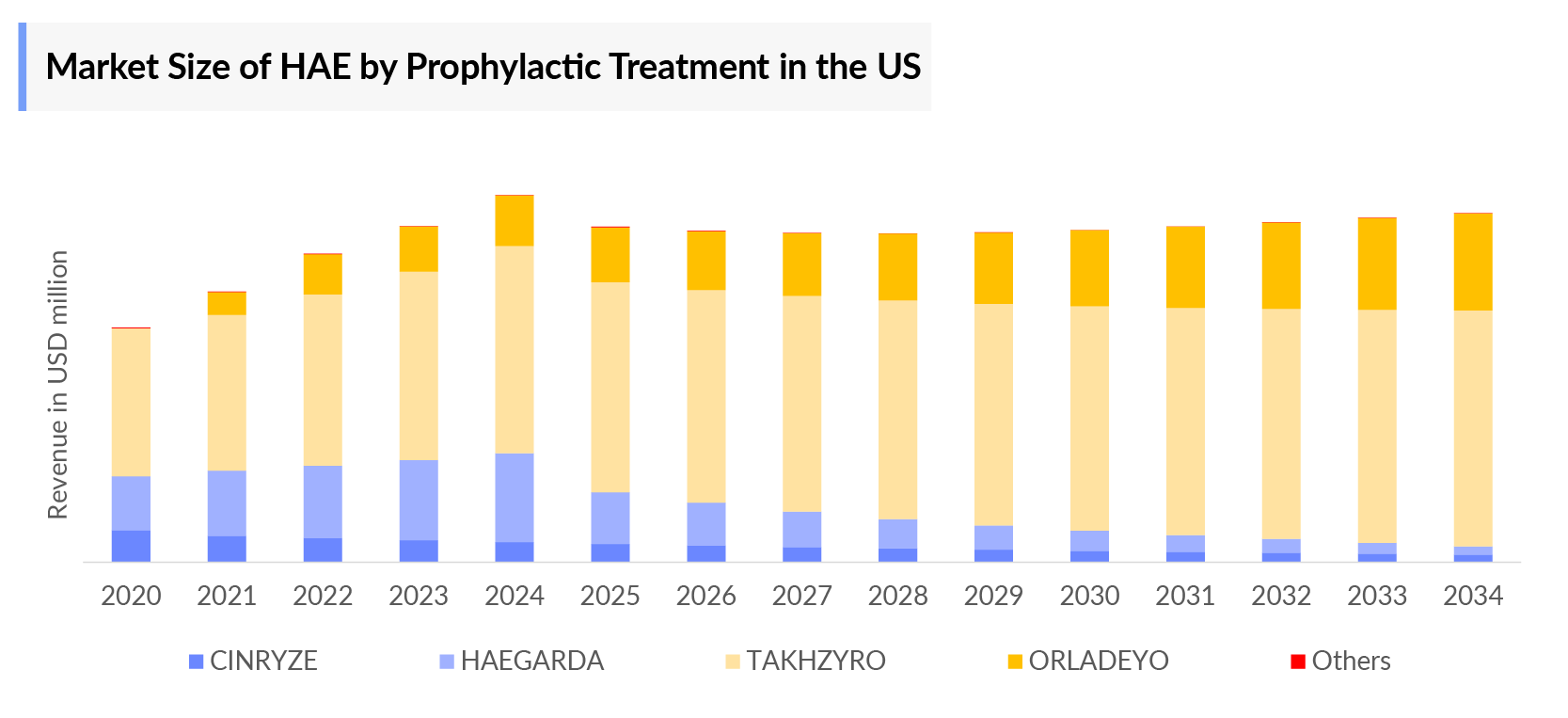
- Treatments for Hereditary angioedema fall into three categories: C1-INH concentrates (plasma-derived (BERINERT) or Recombinant (RUCONEST), kallikrein inhibitor (Ecallantide) and bradykinin receptor antagonist (FIRAZYR). And for the prophylaxis, currently CINRYZE, HAEGARDA and LANADELUMAB. All these therapies are currently approved in the US market.
- Hereditary Angioedema is frequently perceived as affecting females more than males, with approximately 65% of cases occurring in women.
- The Hereditary Angioedema therapeutics market is anticipated to witness a substantial positive shift owing to better uptake of existing drugs, the expected Hereditary angioedema market launch of therapies, and raised awareness.
- The United States accounts for the largest Hereditary angioedema market size ~90%, in comparison to EU4 (Germany, Spain, Italy, France), the United Kingdom, and Japan.
- Hereditary Angioedema pipeline possesses potential drugs like Navenibart (STAR-0215), Donidalorsen, NTLA-2002, sebetralstat and others.
- In November 2024, Ionis Pharmaceuticals, Inc. announced that the U.S. FDA has accepted the New Drug Application (NDA) for donidalorsen, an investigational RNA-targeted treatment for preventing Hereditary Angioedema attacks in adults and pediatric patients aged 12 and older.
- In June 2024, KalVista submitted a New Drug Application (NDA) for U.S. Food and Drug Administration (FDA) review of sebetralstat, a novel investigational oral plasma kallikrein inhibitor for the treatment of HAE attacks in adults and pediatric patients aged 12 years and older.
- In August 2024, Astria Therapeutics announced that it has selected Ypsomed as its partner to develop an autoinjector for STAR-0215.
- In May 2024, Ionis Pharmaceuticals, announced positive results from the Phase III OASIS-HAE and OASISplus studies of donidalorsen for Hereditary Angioedema. The studies showed significant and sustained reductions in monthly HAE attack rates, with over 90% improvement after one year of treatment, whether administered monthly or bi-monthly. Results will be presented at the 2024 European Academy of Allergy and Clinical Immunology (EAACI) Annual Congress in Valencia and published in The New England Journal of Medicine.
DelveInsight's “Hereditary Angioedema Market Insights, Epidemiology and Market Forecast – 2034” report delivers an in-depth understanding of Hereditary Angioedema, historical and forecasted epidemiology as well as the Hereditary Angioedema therapeutics market trends in the United States, EU4 (Germany, Spain, Italy, and France) and the United Kingdom, and Japan.
Hereditary Angioedema market report provides real-world prescription pattern analysis, emerging drugs, market share of individual therapies, and historical and forecasted 7MM Hereditary Angioedema market size from 2020 to 2034. The report also covers current Hereditary Angioedema treatment practices/algorithms and unmet medical needs to curate the best opportunities and assess the market’s underlying potential.
|
Study Period |
2020 to 2034 |
|
Forecast Period |
2024-2034 |
|
Geographies Covered |
|
|
Hereditary Angioedema Market |
|
|
Hereditary Angioedemas Market Size | |
|
Hereditary Angioedema Companies |
Shire, Takeda Pharma, CSL Behring, Pharming Group, BioCryst Pharmaceuticals, Ionis Pharmaceuticals, KalVista Pharmaceuticals, and others. |
|
Hereditary Angioedema Epidemiology Segmentation |
|
Hereditary Angioedema Treatment Market
Hereditary Angioedema Overview, Country-Specific Treatment Guidelines and Diagnosis
Hereditary Angioedema is a rare genetic disorder characterized by recurrent episodes of severe swelling (angioedema) in various parts of the body. The most commonly affected areas are the limbs, face, intestinal tract, and airway. Swelling can occur without a known trigger, but minor trauma or stress may also provoke an attack. Episodes involving the intestinal tract cause severe abdominal pain, nausea, and vomiting, while airway swelling can restrict breathing and lead to life-threatening obstruction. Symptoms typically begin in childhood and worsen during puberty, with the frequency and duration of attacks varying greatly among affected individuals. HAE is broadly divided into two types based on C1 inhibitor (C1-INH) levels in the blood: hereditary angioedema due to C1-INH deficiency (types I and II) and hereditary angioedema with normal C1-INH (type III).
Accurately diagnosing HAE can be challenging due to the rarity of the disease and the need to rule out more common conditions with similar symptoms. The diagnosis is made through a combination of a thorough clinical evaluation, detailed patient history, and blood tests to measure the level and function of C1-INH proteins. In cases of high clinical suspicion and recurrent episodic angioedema of uncertain etiology, genetic testing may be indicated. Measuring C4 and C1-inhibitor levels is a key diagnostic method.
Further details related to country-based variations in diagnosis are provided in the report..
Hereditary Angioedema Treatment
Treatment approaches include medications to prevent attacks and medications to treat Hereditary Angioedema attacks. Preventive Hereditary Angioedema medications may be prescribed by your doctor. In addition to medications, being aware of early warning signs and triggers that can provoke an attack is crucial. Recording information in a diary or journal can help you and your doctor develop a personalized management plan to empower you to lead a full life. Supportive care and medications such as C1 inhibitor, ecallantide, and icatibant are used in the treatment of HAE.
Hereditary Angioedema Epidemiology
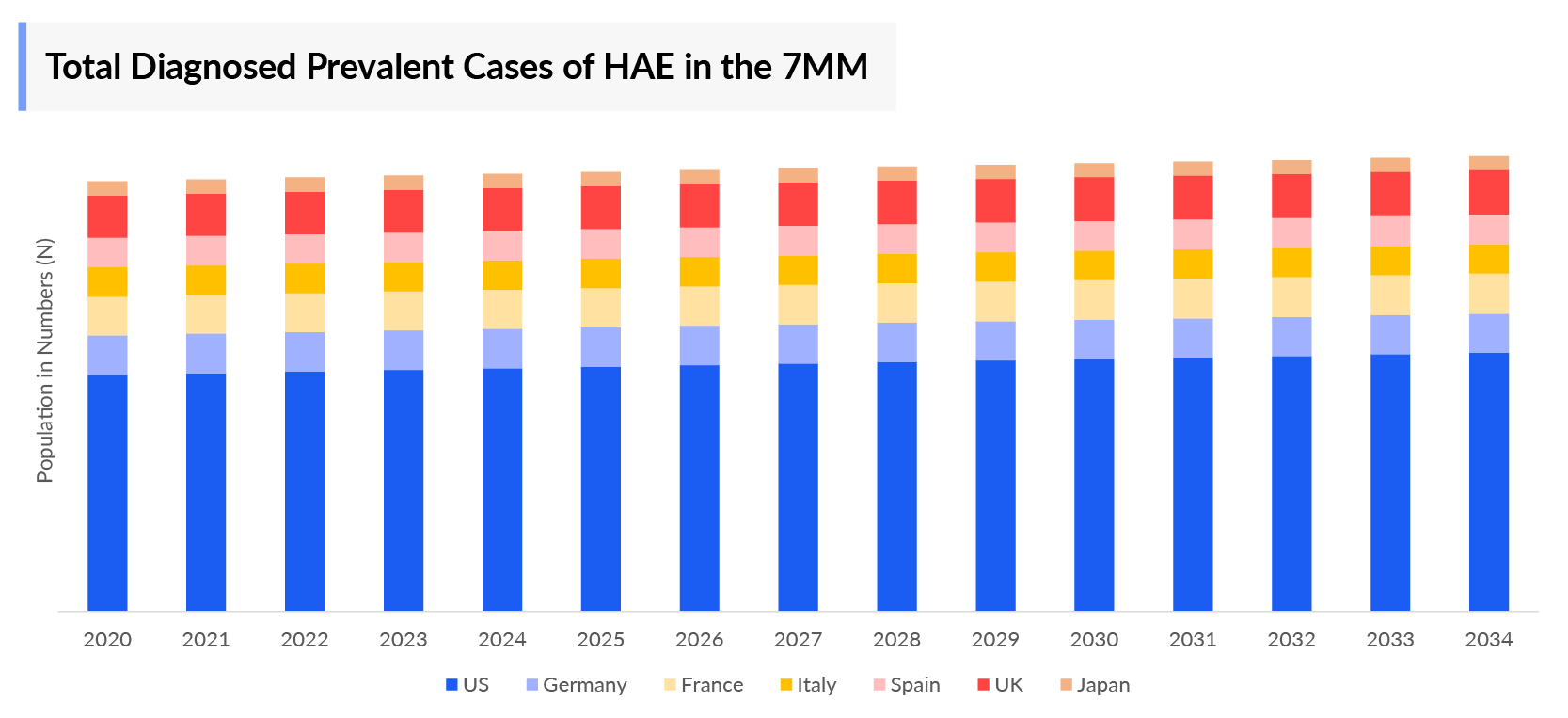
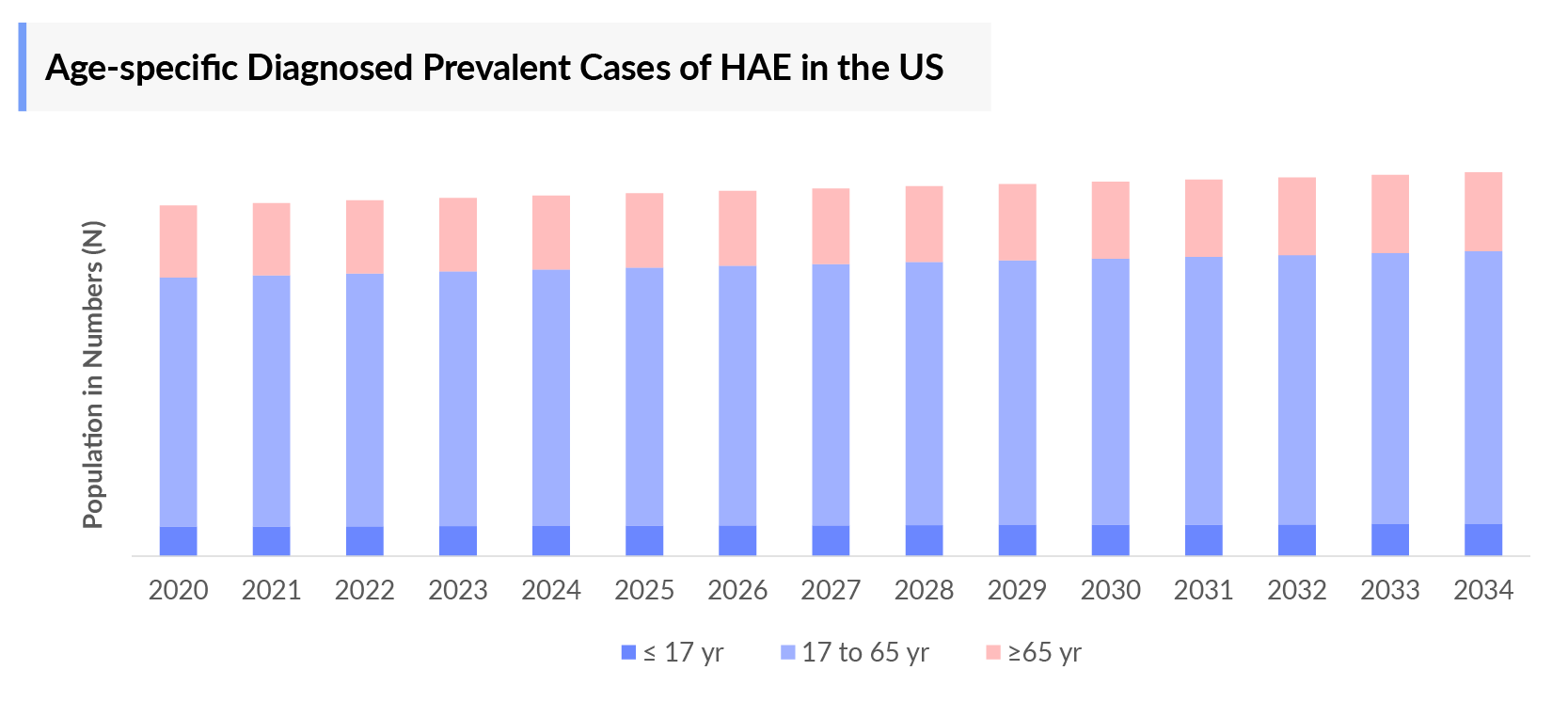
The Hereditary Angioedema epidemiology chapter in the report provides historical as well as forecasted in the 7MM covering the United States, EU4 countries (Germany, France, Italy, and Spain), the United Kingdom, and Japan from 2024 to 2034. The Hereditary Angioedema epidemiology is segmented with detailed insights into total diagnosed prevalent population, type-specific, gender-specific, age-specific, site-specific cases of Hereditary Angioedema.
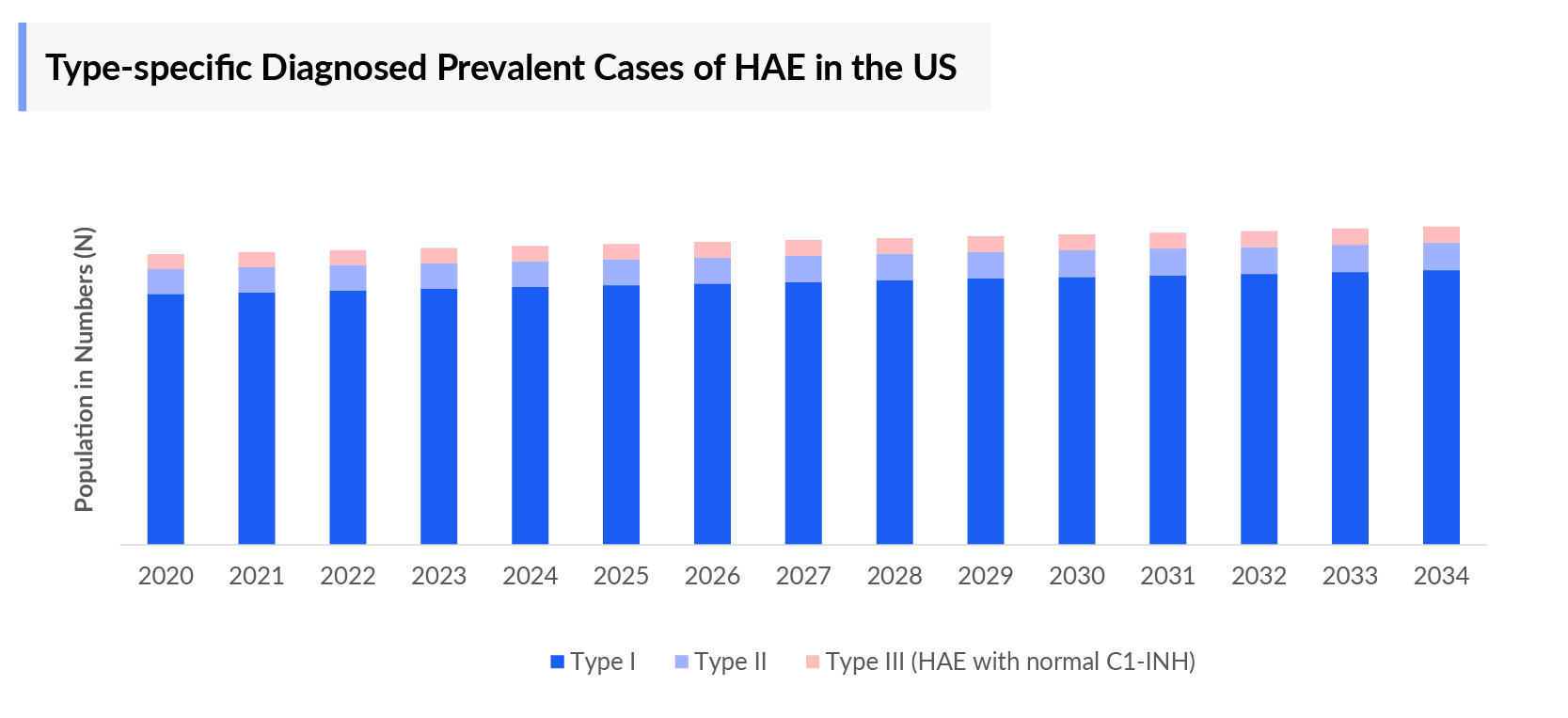
- In 2023, the diagnosed prevalent population of HAE was found to be ~7,000 cases in the US.
- With type I Hereditary Angioedema occupying the maximum diagnosed patient pool in the US, this condition had ~5,500 diagnosed cases in 2023.
- DelveInsight’s analysts have estimated that there were ~1,000 cases diagnosed for the age group 17 to below 65 years in 2023.
Stay Informed on Hereditary Angioedema Epidemiology! Access comprehensive data on demographics and disease burden for effective healthcare planning.
Recent Developments in Hereditary Angioedema Clinical Trials
- In September 2025, following FDA approval of Dawnzera (donidalorsen) for hereditary angioedema (HAE), Piper Sandler reaffirmed an Overweight rating on Ionis Pharmaceuticals and raised its price target to $65, highlighting the company’s strong sales team and potential in the competitive HAE market.
- In August 2025, Ionis Pharmaceuticals (NASDAQ: IONS) announced FDA approval of Dawnzera (donidalorsen) for preventing hereditary angioedema (HAE) attacks in patients aged 12 and older. Dawnzera is the first RNA-targeted therapy for HAE, designed to inhibit plasma prekallikrein (PKK), a key trigger of inflammatory attacks.
- In July 2025, KalVista Pharmaceuticals announced FDA approval of EKTERLY® (sebetralstat), the first oral on-demand treatment for acute hereditary angioedema (HAE) attacks in patients aged 12 and older. EKTERLY offers rapid symptom relief and could transform HAE management as the first new on-demand therapy in over a decade.
- In June 2025, BioCryst Pharmaceuticals, Inc. (Nasdaq: BCRX) announced new long-term efficacy and safety data for ORLADEYO® (berotralstat), used as prophylactic treatment for hereditary angioedema (HAE) in patients of all ages.
- In June 2025, the FDA approved CSL’s Andembry (garadacimab-gxii) for preventing hereditary angioedema (HAE) attacks in patients 12 and older. Phase III trial results showed a 99% reduction in monthly attacks, with 62% of patients attack-free for six months.
- In June 2025, CSL announced FDA approval of ANDEMBRY® (garadacimab-gxii), the first once-monthly subcutaneous treatment targeting factor XIIa to prevent hereditary angioedema (HAE) attacks in patients aged 12 and older.
- In June 2025, KalVista Pharmaceuticals announced that the FDA will miss the June 17 PDUFA goal date for its NDA review of sebetralstat, an oral on-demand treatment for hereditary angioedema, due to workload constraints. A decision is expected within about four weeks.
- In May 2025, BioCryst Pharmaceuticals (Nasdaq: BCRX) reported strong first-quarter results, driven by significant growth in ORLADEYO® (berotralstat) revenue. The company exceeded expectations by moving patients from free drug to paid status faster than anticipated, prompting an upward revision of its annual guidance. BioCryst is now on track to reach peak sales of $1 billion for ORLADEYO, accelerating its path to profitability while beginning to pay down debt and continue advancing its pipeline.
- In November 2024, Ionis Pharmaceuticals, Inc. announced that the U.S. FDA has accepted the New Drug Application (NDA) for donidalorsen, an investigational RNA-targeted treatment for preventing Hereditary Angioedema attacks in adults and pediatric patients aged 12 and older.
Hereditary Angioedema Drug Chapter
The drug chapter segment of the Hereditary Angioedema therapeutics market report encloses a detailed analysis of Hereditary Angioedema marketed drugs and late-stage (Phase III and Phase II) pipeline drugs. It also deep dives into the Hereditary Angioedema clinical trial details, recent and expected market approvals, patent details, the latest news, and recent deals and collaborations.
Marketed Hereditary Angioedema Drugs
Takhzyro: Takeda
Takhzyro (Lanadelumab; DX-2930) is the first of its kind, a fully human monoclonal antibody (mAb) that binds and inhibits the activity of plasma kallikrein, an enzyme that is uncontrolled in people with HAE. Plasma kallikrein is a protease that cleaves high-molecular-weight-kininogen (HMWK) to generate cleaved HMWK (cHMWK) and bradykinin. This potent vasodilator increases vascular permeability resulting in swelling and pain associated with HAE. In patients with HAE due to C1-inhibitor (C1-INH) deficiency or dysfunction, it led to uncontrolled increases in plasma kallikrein activity and results in angioedema attacks. Lanadelumab decreases plasma kallikrein activity to control excess bradykinin generation in patients with HAE. Lanadelumab is produced in Chinese Hamster Ovary (CHO) cells by recombinant DNA technology and available as subcutaneous formulation.
Firazyr: Shire/Takeda
Firazyr (Icatibant) is a selective, competitive bradykinin B2 receptor antagonist indicated for the treatment of acute attacks of Hereditary Angioedema in adults 18 years of age and older. The dysfunctional C1-esterase-inhibition leads to bradykinin production. Bradykinin is a vasodilator which is thought to be responsible for the characteristic HAE symptoms of localized swelling, inflammation, and pain. Icatibant inhibits bradykinin from binding the B2 receptor, with an affinity similar to bradykinin, and thereby treats the clinical symptoms of an acute, episodic attack of HAE. Firazyr is currently approved in 37 countries worldwide, including the countries of the European Union.
Note: Detailed current therapies assessment will be provided in the full report of Hereditary Angioedema...
Emerging Hereditary Angioedema Drugs
Donidalorsen (IONIS-PKK-LRx): Ionis Pharmaceuticals
Donidalorsen, formerly known as IONIS-PKK-LRx, is an investigational LIgand-Conjugated Antisense (LICA) medicine designed to target the production of prekallikrein (PKK), which plays an important role in the activation of inflammatory mediators associated with acute attacks of Hereditary Angioedema. By reducing the production of PKK, donidalorsen could be an effective prophylactic approach to preventing HAE attacks.
In the phase III OASIS-HAE study, donidalorsen administered subcutaneously every 4 weeks (Q4W) showed an 81% lower mean monthly attack rate compared to placebo over weeks 1 to 25 (p<0.001). The Q8W dosing showed a 55% reduction (p=0.004).
Navenibart (STAR-0215): Astria Therapeutics
navenibart (STAR-0215), is a potential best-in-class monoclonal antibody inhibitor of plasma kallikrein designed to provide long-acting, safe, and effective attack prevention for HAE, with potential for dosing once every 3 and 6 months.
Positive initial proof-of-concept results from the ALPHA-STAR Phase Ib/II clinical trial evaluating navenibart in HAE patients demonstrate a favorable safety and tolerability profile, mean monthly attack rate reduction of 90-96% for up to 6 months of follow up, and support both three- (Q3M) and six- month (Q6M) dosing regimens. Based on the positive results, company plan to advance navenibart to Phase III development with trial initiation expected in Q1 2025 and top-line results expected by year-end 2026.
Explore the latest insights into the Hereditary Angioedema pipeline, emerging therapies, and future treatment potential. Stay informed today!
Note: Detailed emerging therapies assessment will be provided in the final report...
|
Therapy Name |
Company Name |
ROA |
MOA |
Phases |
Any Special Status |
|
Navenibart (STAR-0215) |
Astria Therapeutics |
SC |
Inhibitor of plasma kallikrein |
I/II |
Fast Track Designation |
|
Donidalorsen |
Ionis Pharmaceuticals |
SC |
ligand-conjugated antisense oligonucleotide |
III |
N/A |
|
NTLA-2002 |
Intellia Therapeutics |
IV |
CRISPR/Cas9 technology |
I/II |
Orphan Drug Designation and Regenerative Medicine Advanced Therapy (RMAT) Designation |
Hereditary Angioedema Market Outlook
Key players, such as Astria Therapeutics, Ionis Pharmaceuticals, Intellia Therapeutics,and others are evaluating their lead candidates in different stages of clinical development, respectively. They aim to investigate their products for the treatment of Hereditary Angioedema.
- The United States accounts for the highest Hereditary Angioedema market size with ~USD 2,000 million in 2023 and market is expected to increase in forecasted period.
- Three classes of Hereditary Angioedema drugs – C1-INHs, 17 alpha-alkylated androgens, and antifibrinolytics – are currently being used for long-term prophylaxis of HAE type 1 and 2.
- Among EU4 and the UK, France have the highest Hereditary Angioedema market size ~USD 100 million in 2023.
Hereditary Angioedema Drugs Uptake
This section focuses on the uptake rate of potential Hereditary Angioedema drugs expected to be launched in the Hereditary angioedema market during 2024–2034, which depends on the competitive landscape, safety, and efficacy data along with order of entry. It is important to understand that the key Hereditary angioedema companies evaluating their novel therapies in the pivotal and confirmatory trials should remain vigilant when selecting appropriate comparators to stand the greatest chance of a positive opinion from regulatory bodies, leading to approval, smooth launch, and rapid uptake.
Further detailed analysis of emerging therapies drug uptake in the report…
Hereditary Angioedema Pipeline Activities
The Hereditary Angioedema treatment market report provides insights into Hereditary Angioedema clinical trials within Phase III and Phase II stages. It also analyzes key Hereditary angioedema companies involved in developing targeted therapeutics.
Pipeline Development Activities
The Hereditary Angioedema therapeutics market report covers information on collaborations, acquisitions and mergers, licensing, and patent details for Hereditary Angioedema emerging therapies.
KOL Views on Hereditary angioedema
To keep up with the real-world scenario in current and emerging Hereditary Angioedema market trends, we take opinions from Key Industry leaders working in the domain through primary research to fill the data gaps and validate our secondary research. Industry Experts were contacted for insights on the evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake along with challenges related to accessibility.
DelveInsight’s analysts connected with 40+ KOLs to gather insights; however, interviews were conducted with 25+ KOLs in the 7MM. Centers such as University Hospital Frankfurt, Penn State University in Hershey, BioCryst proprietary qualitative research, etc., were contacted. Their opinion helps understand and validate current and emerging treatment patterns of Hereditary Angioedema. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
|
Region |
KOL Views |
|
United States |
"HAE attacks can be serious, painful and frightening, and until recently they required most patients to travel to a clinic or emergency department for treatment, a time-consuming process that can increase the patient’s anxiety, fortunately, three HAE therapies are now indicated for self-administration, which our survey confirmed has the potential to minimize the burden of disease for these patients because when faced with an attack, they can intervene earlier." |
|
spain |
We have diagnosed aproximately 550 patients with HAE due to C1INH deficiency and around 90 with HAE due to coagulation FXII mutations from different regions of Spain, but the prevalence is estimated approximately 5/100.000 |
|
United Kingdom |
“Takhzyro becomes the first self-injected subcutaneous treatment to be approved for use in attack prevention. “HAE is a chronic disease that results in acute attacks of swelling of various body parts and which can be life-threatening. As a physician who treats patients with HAE, I am pleased to have access to a treatment like TAKHZYRO to help prevent HAE attacks,” |
|
Japan |
“Using NAV Vectors to deliver therapeutic antibodies has enormous potential for patients who lack treatments or who are currently underserved by existing therapies, and provides a significant opportunity to expand our pipeline through the application of our AAV-mediated antibody delivery capabilities and expertise to a number of validated and new targets in multiple therapeutic areas and tissues.” |
Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT analysis and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of gaps in disease diagnosis, patient awareness, physician acceptability, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided.
Conjoint Analysis analyzes multiple approved and emerging Hereditary Angioedema therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy.
In efficacy, the trial’s primary and secondary outcome measures are evaluated; one of the most important primary outcome measures is Percentage of Participants with at Least One Treatment-emergent Adverse Event (TEAE), Graded by Severity
Further, the therapies’ safety is evaluated wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Hereditary Angioedema Market Access and Reimbursement
The Hereditary Angioedema market report further provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of currently used therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Scope of the Hereditary Angioedema Market Report
- The Hereditary Angioedema market report covers a segment of key events, an executive summary, descriptive overview of Hereditary Angioedema, explaining its causes, signs and symptoms, pathogenesis, and currently available therapies.
- Comprehensive insight has been provided into the epidemiology segments and forecasts, the future growth potential of diagnosis rate, and disease progression along with country specific treatment guidelines.
- Additionally, an all-inclusive account of both the current and emerging Hereditary angioedema therapies, along with the elaborative profiles of late-stage and prominent therapies, will have an impact on the current treatment landscape.
- A detailed review of the Hereditary Angioedema market, historical and forecasted market size, market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The Hereditary angioedema market report provides an edge while developing business strategies, by understanding trends, through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help in shaping and driving the 7MM Hereditary Angioedema market.
Hereditary Angioedema Market Report Insights
- Hereditary Angioedema Patient Population
- Hereditary Angioedema Therapeutic Approaches
- Hereditary Angioedema Pipeline Analysis
- Hereditary Angioedema Market Size
- Hereditary Angioedema Market Trends
- Existing and future Hereditary Angioedema Market Opportunity
Hereditary Angioedema Market Report Key Strengths
- Eleven Years Forecast
- 7MM Coverage
- Hereditary Angioedema Epidemiology Segmentation
- Inclusion of Country specific treatment guidelines
- KOL’s feedback on approved and emerging therapies
- Key Cross Competition
- Hereditary Angioedema Conjoint analysis
- Hereditary Angioedema Drugs Uptake
- Key Hereditary Angioedema Market Forecast Assumptions
Hereditary Angioedema Market Report Assessment
- Current Hereditary Angioedema Treatment Practices
- Hereditary Angioedema Unmet Needs
- Hereditary Angioedema Pipeline Product Profiles
- Hereditary Angioedema Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
- Hereditary Angioedema Market Drivers
- Hereditary Angioedema Market Barriers
FAQs
- What is the growth rate of the 7MM Hereditary Angioedema treatment market?
- What was the Hereditary Angioedema market size, the market size by therapies, market share (%) distribution in 2020, and what would it look like in 2034? What are the contributing factors/key catalysts for this growth?
- Is there any unexplored patient setting that can open the window for growth in the future?
- What are the pricing variations among different geographies for approved and off-label Hereditary Angioedema therapies?
- How would the market drivers, barriers, and future opportunities affect the Hereditary Angioedema market dynamics and subsequent analysis of the associated trends? Although multiple expert guidelines recommend testing for targetable mutations before therapy initiation, why do barriers to testing remain high?
- What are the current and emerging options for the treatment of Hereditary Angioedema?
- How many companies are developing therapies for the treatment of Hereditary Angioedema?
- What are the recent novel therapies, targets, mechanisms of action, and technologies developed to overcome the limitations of existing therapies?
- Patient/physician acceptability in terms of preferred treatment options as per real-world scenarios?
- What are the country-specific accessibility issues of expensive, recently approved Hereditary Angioedema therapies?
Reasons to buy Hereditary Angioedema Market Forecast Report
- The Hereditary Angioedema market report will help in developing business strategies by understanding the latest trends and changing treatment dynamics driving the Hereditary Angioedema Market.
- Insights on patient burden, Hereditary angioedema prevalence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years
- Understand the existing Hereditary Angioedema market opportunities in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
- Identifying strong upcoming Hereditary angioedema companies in the Hereditary Angioedema market will help devise strategies to help get ahead of competitors.
- Detailed analysis and ranking of class-wise potential current and emerging therapies under the conjoint analysis section to provide visibility around leading classes.
- Highlights of access and reimbursement policies of approved therapies, barriers to accessibility of expensive off-label therapies, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing market so that the upcoming Hereditary Angioedema companies can strengthen their development and launch strategy.