Hidradenitis Suppurativa Market
- The Hidradenitis Suppurativa Treatment Market Size is anticipated to grow with a significant CAGR during the study period (2020-2034).
- To put the Hidradenitis Suppurativa Market opportunity into perspective for a condition that affects roughly 1% of the population, it is clear that, with the right strategy, it has the potential to be very profitable.
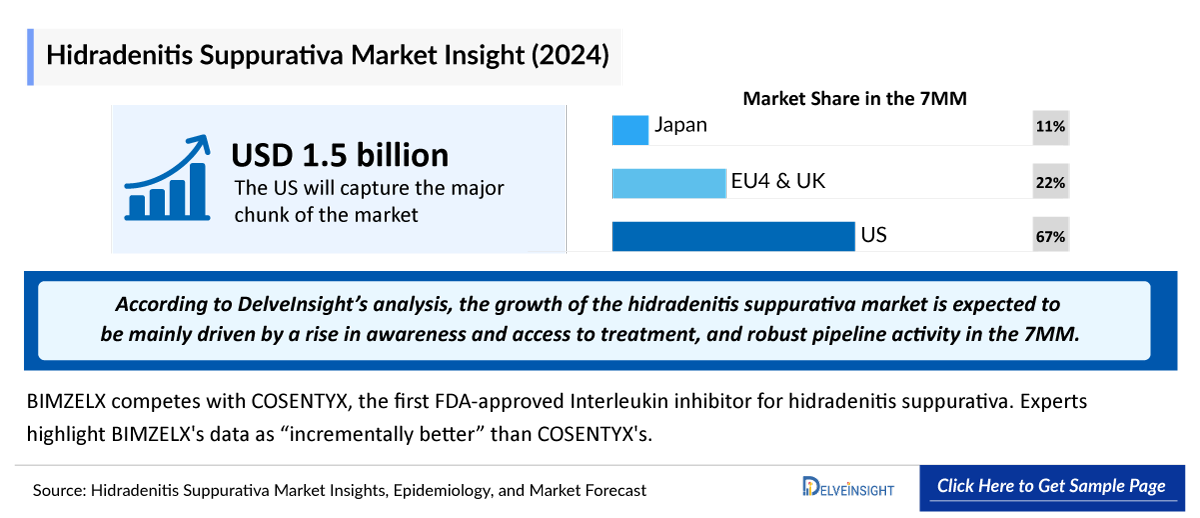
- In 2023, Hidradenitis Suppurativa Market Size was nearly USD ~1,400 million in the 7MM. The largest market size was captured by the US.
- In the 7MM, the US accounted for the highest number of Hidradenitis Suppurativa Prevalence Cases with nearly 2,881,900 in 2023.
- Hidradenitis suppurativa management typically involves a multidisciplinary approach, including surgery, topical or systemic antibiotics, corticosteroids, and procedural interventions like deroofing and excision.
- Biologics have become crucial Hidradenitis Suppurativa treatment landscape options for moderate-to-severe cases, potentially altering treatment approaches. Currently, HUMIRA (adalimumab) and COSENTYX (secukinumab) are the only biologics approved by the US FDA for treating hidradenitis suppurativa. BIMZELX (bimekizumab), as of now, is only approved in Europe for treating hidradenitis suppurativa; however, the US FDA has accepted for review the sBLA for BIMZELX in April 2024 for the treatment of hidradenitis suppurativa.
- HUMIRA, AbbVie’s flagship product, is a classic example of maintaining a market monopoly for an extended period. However, the introduction of biosimilars has significantly affected HUMIRA’s sales in the 7MM. In Europe and Japan, HUMIRA biosimilars were launched in 2018 and 2021. In January 2023, AMJEVITA became the first HUMIRA biosimilar to be launched in the US.
- Although BIMZELX is more effective, COSENTYX has the first-mover advantage. According to reports, COSENTYX is experiencing strong launch momentum. It is worth highlighting that BIMZELX's efficacy is differentiated to both HUMIRA and COSENTYX. Although Novartis' clearance has allowed COSENTYX some leeway in treating hidradenitis suppurativa, UCB’s BIMZELX, which yielded "incrementally better" outcomes than COSENTYX, might pose a serious threat to the medication in the near future.
- The hidradenitis suppurativa market is experiencing rapid growth, driven by an influx of pipeline therapeutics. The diverse pipeline includes treatments targeting interleukins (such as IL-17, IL-36, and IL-1a/b), PDE4 inhibitors, JAK inhibitors, and CXCR1/CXCR2 inhibitors.
- For moderate-to-severe and biologic experiences, many therapies, including Izokibep (ACELYRIN), Sonelokimab (MoonLake Immunotherapeutics), Povorcitinib (Incyte Corporation), Spesolimab (Boehringer Ingelheim), Lutikizumab (AbbVie), Eltrekibart (Eli Lilly) and others are being investigated.
- Povrocitinib may become the first oral medication to enter this rapidly expanding Hidradenitis Suppurativa market size . The outcomes of Povrocitinib outperform those of RINVOQ.
- Among the few top data presented on hidradenitis suppurativa at the American Academy of Dermatology 2024 conference includes Incyte Corporation’s Phase II study evaluating ruxolitinib 1.5% cream for hidradenitis suppurativa drugs and 24-week topline results from the Phase II MIRA trial evaluating sonelokimab.
- Since nanobodies may target many pathways and have superior tissue penetration, there is a lot of enthusiasm for nanobodies because of their potential to treat hidradenitis suppurativa. The potential for Sonelokimab to replace COSENTYX exists if Moonlake's Phase II findings are similarly replicated in Phase III.
- Despite the considerable impact on patients' well-being, the mild segment is still untapped without any approved therapy and only two emerging therapies, namely, orismilast and ruxolitinib cream.
Request for Unlocking the Sample Page of the "Hidradenitis Suppurativa Drugs Market"
Key Factors Driving Hidradenitis Suppurativa Market
Increasing Hidradenitis Suppurativa Prevalence and Diagnosis Rates: The global prevalence of HS is rising, with estimates suggesting 1–4% of the population may be affected. Growing disease awareness among patients and healthcare providers has led to higher diagnosis rates and increased demand for treatment.
Emerging Therapeutic Options for ADA-Refractory Hidradenitis Suppurativa: Anti-IL-17 and anti-IL-1α inhibition are promising therapeutic options for ADA-refractory moderate-to-severe hidradenitis suppurativa.
Emergence of Novel Drug Classes: The hidradenitis suppurativa market has a diverse pipeline targeting Interleukins (i.e., IL-17, IL-36), anti-TNF, JAKi, and anti-complement factors, which are contributing to the progression of the market.
Emergence of Oral Therapies Enhancing Patient Convenience: Emerging therapies are coming with patient-convenient RoA, such as RINVOQ, povorcitinib, and orismilast, which are given by oral route.
Next-Generation Hidradenitis Suppurativa Therapies Shaping the Future: Some of the drugs in the pipeline include Eltrekibart (Eli Lilly), Sonelokimab (MoonLake Immunotherapeutics), Brepocitinib (Priovant Therapeutics), Spesolimab (Boehringer Ingelheim), Orismilast (UNION Therapeutics), Povorcitinib (Incyte Corporation), and others.
DelveInsight’s " Hidradenitis Suppurativa Market Insight, Epidemiology, and Market Forecast – 2034" report delivers an in-depth understanding of hidradenitis suppurativa, historical and forecasted epidemiology as well as the hidradenitis suppurativa therapeutics market trends in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
The Hidradenitis Suppurativa Drugs Market report provides current treatment practices, emerging drugs, hidradenitis suppurativa share of individual therapies, and current and forecasted hidradenitis suppurativa market size from 2020 to 2034, segmented by seven major markets. The Hidradenitis Suppurativa market size report also covers hidradenitis suppurativa treatment practices/algorithms and unmet medical needs to curate the best of the opportunities and assess the underlying potential of the market.
|
Study Period |
2020 to 2034 |
|
Forecast Period |
2024-2034 |
|
Geographies Covered |
|
|
Hidradenitis Suppurativa Drugs Market |
|
|
Hidradenitis Suppurativas Market Size | |
|
Hidradenitis Suppurativa Companies |
|
|
Hidradenitis Suppurativa Epidemiology Segmentation |
|
Hidradenitis Suppurativa Treatment Market
Hidradenitis suppurativa is a chronic condition characterized by swollen, painful lesions occurring in the armpit, groin, anal and breast regions. It is a painful and long-term skin condition causing abscesses and scarring on the skin. Affected patients may present with acute abscesses, but the condition often progresses to a chronic state with persistent pain, sinus tract fistula formation, and scarring. It is usually associated with depression and severe overall impairment of quality of life (QoL), exceeding other skin disorders heavily impacting QoL, such as psoriasis, atopic dermatitis, alopecia, and acne.
There are several scoring systems for the assessment of disease severity of hidradenitis suppurativa, including Hurley staging, Physician’s Global Assessment (PGA), the modified Sartorius score (MSS), and hidradenitis suppurativa Severity Index (HSSI). Based on Hurley staging, the disease is characterized by three clinical stages, i.e., Hurley stages 1, 2, and 3.
Hidradenitis Suppurativa Diagnosis
The diagnosis is primarily clinical, based on symptoms reported by the patient and signs observed by the physician. No pathognomonic test exists, and biopsy is rarely required, especially in well-developed lesions. Primary diagnosis of hidradenitis suppurativa involves identification of the disease and assessment of its comorbidities. Fulfilment of three criteria is necessary for the diagnosis of hidradenitis suppurativa:
Typical lesions: deep-seated, painful nodules
Characteristic distribution: Typical anatomical predilection (i.e., axillae, groins, perineal and perianal regions, buttocks, infra-mammary and inter-mammary folds)
Recurrence: Chronicity and recurrence of lesions.
In certain situations, a biopsy is required to confirm the diagnosis, such as in the atypical cases of perianal Crohn's disease, tuberculous ulcer, and carcinoma. The association with spinocellular carcinoma, in case of long-term progress, is very rare.
Hidradenitis Suppurativa Treatment
The overall treatment goals for hidradenitis suppurativa treatment landscape include alleviating lesion-related symptoms (e.g., pain), reducing recurrence frequency and new lesion formation, and preventing disease and comorbidity progression.
Based upon the published guidelines for hidradenitis suppurativa, the first line of therapy includes topical clindamycin, oral clindamycin/rifampicin, tetracycline, and SC adalimumab. A similar approach was taken for the second-line therapies, including zinc gluconate, resorcinol, intralesional corticosteroids, infliximab, acitretin, and etretinate. Third-line therapies included colchicine, botulinum toxin, isotretinoin, dapsone, cyclosporine, and hormones. Surgical evaluated therapies included individual lesions excision, total excision of lesions surrounding hair-bearing skin, secondary intention healing, primary closure, reconstructive with skin grafting and NPWT reconstruction with flap, deroofing, carbon dioxide laser therapy, laser therapy, and intense pulsed light.
The preferred biologic agent for hidradenitis suppurativa is HUMIRA (adalimumab), as it is backed by extensive research. Other approved therapies include COSENTYX and BIMZELX to treat moderate-to-severe hidradenitis suppurativa in adults..
Hidradenitis Suppurativa Epidemiology
The hidradenitis suppurativa epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by the Total Prevalent Cases of Hidradenitis Suppurativa, Total Diagnosed Prevalent Cases of Hidradenitis Suppurativa, Gender-Specific Prevalent Cases of Hidradenitis Suppurativa, Age-specific Prevalent Cases of Hidradenitis Suppurativa, Stage-specific Prevalent cases of Hidradenitis Suppurativa, and Treated Prevalent Cases of Hidradenitis Suppurativa in the 7MM market covering the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan from 2020 to 2034.
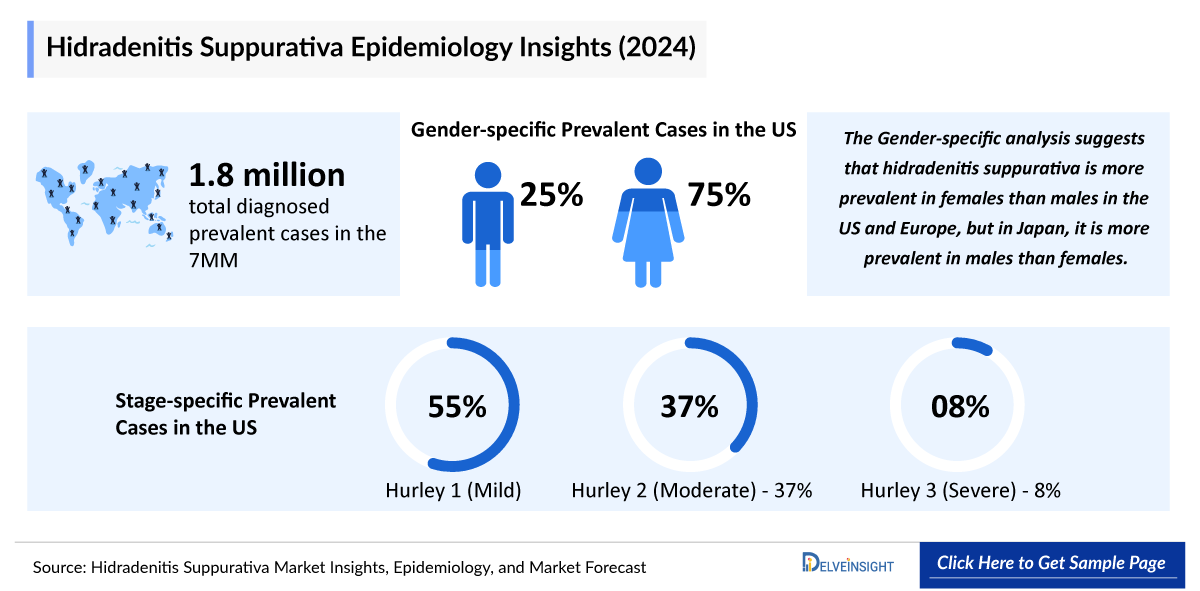
- In 2023, the total Hidradenitis Suppurativa Prevalence Cases in the 7MM were nearly 6.2 million. These cases are anticipated to increase by 2034.
- In the 7MM, the US accounted for the highest number of Hidradenitis Suppurativa Diagnosed Prevalent Cases with nearly 877,600 in 2023.
- Among EU4 and the UK, Germany accounted for the highest number of Hidradenitis Suppurativa Diagnosed Prevalent Cases whereas Spain occupied the bottom of the ladder.
- In the US, EU4 and the UK, hidradenitis suppurativa was seen to be slightly more common in females (~75%) than males (~25%). Conversely, in Japan, males represent nearly 70% of the total cases, while females account for around 30%.
- The age-specific data revealed that hidradenitis suppurativa was most prevalent in the age group of 30–39 years, which was nearly 26% of the total diagnosed cases of hidradenitis suppurativa in the US.
- Among all, Hurley Stage I was observed to be most prevalent, with approximately 480,000 cases in 2023 in the US.
Hidradenitis Suppurativa Drugs Market Chapters
The Hidradenitis Suppurativa drugs market chapter segment of the Hidradenitis Suppurativa Market Report encloses a detailed analysis of the late-stage (Phase III and Phase II) and early-stage (Phase I/II) Hidradenitis Suppurativa pipeline drugs. The current key Hidradenitis Suppurativa Companies for emerging drugs and their respective drug candidates include Incyte Corporation (Povorcitinib), Acelyrin (Izokibep), AbbVie (Upadacitinib; Lutikizumab), MoonLake Immunotherapeutics (Sonelokimab), UNION therapeutics (Orismilast), Eli Lilly (Eltrekibart), Incyte Corporation (Ruxolitinib 1.5% Cream), and others. The drug chapter also helps understand the hidradenitis suppurativa clinical trials details, expressive pharmacological action, agreements and collaborations, approval, and patent details, and the latest Hidradenitis Suppurativa prevalence news and press releases.
Hidradenitis Suppurativa Marketed Drugs
- HUMIRA (adalimumab): AbbVie/Eisai
HUMIRA (adalimumab) is a recombinant human IgG1 monoclonal antibody specific for human tumor necrosis factor (TNF). In September 2015, the US FDA approved HUMIRA for the treatment of moderate-to-severe hidradenitis suppurativa in adults, and later in October 2018, for patients =12 years of age as well. The recommended dose of HUMIRA for adult patients with hidradenitis suppurativa is an initial dose of 160 mg (given in 1 day or split over 2 consecutive days), followed by 80 mg 2 weeks later. In Europe and Japan, HUMIRA biosimilars were launched in 2018 and 2021. In September 2016, AMJEVITA became the first FDA-approved biosimilar for HUMIRA. Then, in January 2023, it became the first HUMIRA biosimilar to be launched in the US.
BIMZELX (Bimekizumab): UCB Biopharma
Bimekizumab is the first humanized monoclonal IgG1 antibody that potently and selectively neutralizes both IL-17A and IL-17F, two key cytokines driving inflammatory processes. In April 2024, UCB announced that the European Commission granted marketing authorization for BIMZELX for the treatment of active moderate-to-severe hidradenitis suppurativa in adults with an inadequate response to conventional systemic hidradenitis suppurativa therapy. The approval follows a positive opinion issued in March 2024 by the Committee for Medicinal Products for Human Use of the EMA. In addition, in April 2024, the US FDA accepted for review the sBLA for BIMZELX for the treatment of adults with moderate-to-severe hidradenitis suppurativa. The drug has also been filed with PMDA in Japan for treating hidradenitis suppurativa.
Hidradenitis Suppurativa Emerging Drugs
- Povorcitinib (INCB054707): Incyte Corporation
Povorcitinib, developed by Incyte Corporation, is a selective JAK1 inhibitor that effectively reduces abscesses and inflammatory nodules to treat patients with moderate-to-severe hidradenitis suppurativa. This therapeutic molecule is administered orally. Currently, the company is investigating this molecule in three different Phase III clinical trials (STOP-HS studies) for moderate-to-severe hidradenitis suppurativa. The company expects the Phase III STOP-HS trial data in 2025. In addition, the company also anticipates the potential launch of povorcitinib for hidradenitis suppurativa by 2026-2027.
- Sonelokimab (M1095): MoonLake Immunotherapeutics
Sonelokimab (M1095) is an investigational ~40 kDa humanized nanobody consisting of three VHH domains covalently linked by flexible glycine-serine spacers. Nanobodies represent a new generation of antibody-derived targeted therapies. With two domains, sonelokimab selectively binds with high affinity to IL-17A and IL-17F, thereby inhibiting the naturally occurring IL-17A/A, IL-17A/F, and IL-17F/F dimers.
The Hidradenitis Suppurativa market companies have already completed a Phase II trial of sonelokimab for treating moderate-to-severe hidradenitis suppurativa and is currently evaluating the drug in two Phase III Hidradenitis Suppurativa clinical trials. The 24-week topline results were presented at the AAD 2024 annual meeting. The initiation of this Phase III program follows the announcement in February 2024 of the successful outcome of MoonLake’s end-of-Phase II interactions with the US FDA, as well as positive feedback from the EMA, supporting MoonLake’s proposed approach for advancing its Phase III program in hidradenitis suppurativa. The Phase III VELA program is expected to enroll 800 patients across VELA-1 and VELA-2. This is the first Phase III program in hidradenitis suppurativa to use the higher clinical response level of HiSCR75 as the primary endpoint. As per the company, the topline primary endpoint readout of the Phase III VELA trial at Week 16, together with data on other endpoints, is expected as of mid-2025.
Hidradenitis Suppurativa Drugs Market Class Insight
Hidradenitis suppurativa is treated using a variety of therapeutic classes tailored to its severity. Topical treatments, including antibiotics and antiseptics, are often used for milder cases. Systemic therapies, such as oral antibiotics, hormonal treatments, and retinoids, are utilized for more extensive disease. Biologic therapies, particularly TNF-alpha inhibitors and interleukin inhibitors, are effective for moderate-to-severe hidradenitis suppurativa. Immunosuppressants like cyclosporine and methotrexate are also options. The diverse Hidradenitis Suppurativa pipeline includes treatments targeting interleukins (such as IL-17, IL-36, and IL-1a/b), PDE4 inhibitors, JAK inhibitors, and CXCR1 and CXCR2 inhibitors.
Hidradenitis Suppurativa Market Outlook
As there is no cure for hidradenitis suppurativa, early diagnosis, and treatment help prevent the disease from getting worse and forming additional scars, and the current treatment approaches depend on the severity and clinical staging of the disease. Regarding pharmacological treatment market options, the following therapies are available: topical and systemic antibiotics, corticosteroids, hormonal therapy, systemic retinoids, zinc supplements, and immunosuppressive agents, including biologics. The mainstay of medical treatment of mild disease involves antibacterial washes and topical antibiotics. Acute flares may be managed by intralesional corticosteroids and/or minor surgical procedures. Oral therapies for mild-to-moderate hidradenitis suppurativa include extended courses of broad-spectrum antibiotics and systemic retinoids. In off-label therapies, the commonly used antibiotics are clindamycin, rifampicin, and tetracycline, as these antibiotics have shown their efficacy in the studies.
Currently, the established therapies in the market include BIMZELX (bimekizumab), COSENTYX (secukinumab), and HUMIRA (adalimumab). HUMIRA was completely dominating the market until 2023, even though its composition-of-matter patent expired in December 2016 in the US; AbbVie has stronger surrounding patents stateside than in Europe, giving them more layers of defense on the biologic’s exclusivity. However, in June 2023, the European Commission approved COSENTYX, an IL-17A monoclonal antibody, for use in adults with active moderate-to-severe hidradenitis suppurativa and an inadequate response to conventional systemic hidradenitis suppurativa therapy. The drug was later approved by the US FDA in October 2023 to treat moderate-to-severe hidradenitis suppurativa in adults. Recently, in April 2024, UCB announced that the European Commission granted marketing authorization for BIMZELX for the treatment of active moderate-to-severe hidradenitis suppurativa in adults with an inadequate response to conventional systemic hidradenitis suppurativa therapy.
Sadaf Javed, Manager of Forecasting and Analytics at DelveInsight, commented that dual- and trispecific agents are present in the emerging pipeline, such as BIMZELX, Sonelokimab, and Lutikizumab, which could improve patient outcomes.
The emerging Hidradenitis Suppurativa pipeline therapies includes promising candidates such as povorcitinib (INCB054707), Spesolimab (BI 655130), Lutikizumab, Eltrekibart, Orismilast, ruxolitinib cream, Izokibep, Sonelokimab, and others. The Hidradenitis suppurativa Drugs Market is currently underserved. It is anticipated that similar to rheumatoid arthritis, hidradenitis suppurativa has a sufficiently large market to support the co-existence of several blockbuster medications.
If the efficacy of oral drugs is largely comparable to SC injections, there may be a preference for oral therapies in this underappreciated market.
- The US accounted for the largest Hidradenitis Suppurativa Market Size in the 7MM, with nearly USD 1,100 million in 2023.
- Among EU4 and the UK, Germany accounted for the maximum Hidradenitis Suppurativa Market Size in 2023, while Spain occupied the bottom of the ladder.
- Patient share of overall adalimumab is also expected to decline due to the recent entry of COSENTYX and the upcoming BIMZELX.
- In the US, by 2034, among all the therapies, the highest revenue is expected to be generated from BIMZELX.
Recent Developments in the Hidradenitis Suppurativa Market
-
In January 2026, MoonLake Immunotherapeutics provided an update following positive feedback from the US Food and Drug Administration (FDA) on the clinical evidence strategy for sonelokimab in hidradenitis suppurativa, following a Type B meeting requested by the company.
-
In November 2025, MoonLake Immunotherapeutics announced key upcoming clinical milestones for sonelokimab in hidradenitis suppurativa. The company expects to report 52-week data from the Phase III VELA-1 and VELA-2 trials in adult hidradenitis suppurativa in Q2 2026. In addition, the primary endpoint readout from the Phase III VELA-TEEN trial, evaluating sonelokimab in adolescent patients with hidradenitis suppurativa, is anticipated in Q2 2026.
-
In September 2025, Incyte presented new 24-week interim data evaluating the safety and efficacy of povorcitinib from the pivotal Phase III STOP-HS clinical trial program in adult patients (≥18 years) with moderate to severe hidradenitis suppurativa at the European Association of Dermatology and Venereology (EADV) 2025 Congress.
-
In March 2025, Incyte announced positive topline results from its pivotal Phase III STOP-HS clinical trial program evaluating the safety and efficacy of povorcitinib.
Hidradenitis Suppurativa Drugs Uptake
This section focuses on the rate of uptake of the potential Hidradenitis Suppurativa drugs expected to be launched in the market during the study period. The analysis covers hidradenitis suppurativa market uptake by drugs, patient uptake by therapies, and sales of each drug. The probability of success in the case of emerging drugs in the case of hidradenitis suppurativa is low, considering the lack of data available on proof of concept studies. The emerging class in hidradenitis suppurativa treatment landscape involves IL-17 inhibitors, with dual inhibition of IL-17A and IL-17F showing the most promise. Clinical data indicates that COSENTYX (secukinumab), which targets only IL-17A, is less effective than BIMZELX (bimekizumab), which inhibits both IL-17A and IL-17F. This underscores the pivotal role of IL-17 cytokines and demonstrates the potential of this class to have a strong uptake.
For mild to moderate hidradenitis suppurativa, ruxolitinib is expected to have a better uptake compared to orismilast, as ruxolitinib cream has demonstrated superior efficacy and safety.
Hidradenitis Suppurativa Pipeline Development Activities
The Hidradenitis Suppurativa pipeline segment report provides insights into different Hidradenitis Suppurativa clinical trials within Phase III, Phase II, and Phase I/II stage. It also analyzes key Hidradenitis Suppurativa Companies involved in developing targeted therapeutics.
Development Activities
The Hidradenitis Suppurativa pipeline segment report covers detailed information on collaborations, acquisitions and mergers, licensing, and patent details for hidradenitis suppurativa emerging therapies.
KOL Views
To keep up with current Hidradenitis Suppurativa Market Size, we take KOLs and SMEs' opinions working in the domain through primary research to fill the data gaps and validate our secondary research. Some of the leaders like MD, Professor and Vice Chair of the Department of Dermatology and Director, PhD, and others. Their opinion helps to understand and validate current and emerging therapies and treatment patterns or hidradenitis suppurativa market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the Hidradenitis Suppurativa Therapeutics market and the Hidradenitis Suppurativa Unmet Needs.
Delveinsight’s analysts connected with 30+ KOLs to gather insights; however, interviews were conducted with 10+ KOLs in the 7MM. Centers such as the Department of Dermatology, CHA Bundang Medical Center, Department of Dermatology & Academic Wound Healing, Cardiff University, University of California, University of London, etc., were contacted. Their opinion helps understand and validate hidradenitis suppurativa epidemiology and market trends.
Hidradenitis Suppurativa Medication Market: Qualitative Analysis
We perform qualitative and Hidradenitis Suppurativa Pipeline Intelligence analysis using various approaches, such as SWOT and conjoint analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving treatment landscape.
The analyst analyzes multiple emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. In efficacy, the trial’s primary and secondary outcome measures are evaluated. Further, the therapies’ safety is evaluated wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials.
Hidradenitis Suppurativa Medication Market Access and Reimbursement
HUMIRA (adalimumab) – a complex medication – is an expensive drug that costs about USD 7,389 for two SC kits (10 mg/0.1 mL); possessing insurance or a discount card aids the patient in paying less. Most insurance plans cover HUMIRA, but individual plans may vary in the coverage range. Insurance can lower the out-of-pocket price of HUMIRA from about USD 7,389 to approximately USD 5,000 per month. Some people who receive employer/retiree commercial prescription coverage will be eligible for a HUMIRA Complete Savings Card. The HUMIRA Complete Savings card is only available to people with private insurance coverage from their employer or to people who have purchased insurance coverage directly from insurance companies. Co-pay assistance is unavailable for people who receive prescription reimbursement through federal, state, or government-funded insurance programs.
COSENTYX Connect is a free, personalized support program for people taking or considering COSENTYX. The goal is to make the COSENTYX experience as easy, affordable, and convenient as possible and pay as little as USD 0 co-pay if eligible. COSENTYX Connect Co-Pay Program provides up to USD 16,000 annually for the cost of COSENTYX and up to USD 150 per infusion (up to USD 1,950 annually) for the cost of administration.
Further detailed analysis will be provided in the report….
Hidradenitis Suppurativa Therapeutics Market Report Scope
- The Hidradenitis Suppurativa market size report covers a descriptive overview of hidradenitis suppurativa, explaining its causes, signs and symptoms, pathogenesis, and currently available therapies.
- Comprehensive insight has been provided into hidradenitis suppurativa epidemiology and treatment.
- Additionally, an all-inclusive account of both the current and emerging therapies for hidradenitis suppurativa is provided, along with the assessment of new therapies that will have an impact on the current Hidradenitis Suppurativa treatment market landscape.
- A detailed review of the hidradenitis suppurativa therapeutics market, historical and forecasted, is included in the report, covering the 7MM drug outreach.
- The Hidradenitis Suppurativa therapeutics market report provides an edge while developing business strategies by understanding trends shaping and driving the 7MM hidradenitis suppurativa market.
Hidradenitis Suppurativa Therapeutics Market Report Insights
- Patient-based Hidradenitis Suppurativa Market Forecasting
- Hidradenitis Suppurativa Therapeutic Approaches
- Hidradenitis Suppurativa Pipeline Drugs Analysis
- Hidradenitis Suppurativa Market Size
- Hidradenitis Suppurativa Market Trends
- Hidradenitis Suppurativa Therapeutics Market Opportunities
- Impact of Upcoming Hidradenitis Suppurativa Therapies
Hidradenitis Suppurativa Therapeutics Market Report Key Strengths
- 11 Years Hidradenitis Suppurativa Market Forecast
- The 7MM Coverage of Hidradenitis Suppurativa Drugs Market
- Hidradenitis Suppurativa Epidemiology Segmentation
- Key Cross Competition
- Highly Analyzed Hidradenitis Suppurativa Therapeutics Market
- Hidradenitis Suppurativa Drugs Uptake
Hidradenitis Suppurativa Therapeutics Market Report Assessment
- Current Hidradenitis Suppurativa Treatment Market Practices
- Hidradenitis Suppurativa Unmet Needs
- Hidradenitis Suppurativa Pipeline Drugs Analysis Profiles
- Hidradenitis Suppurativa Therapeutics Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
- Hidradenitis Suppurativa Market Drivers
- Hidradenitis Suppurativa Market Barriers
FAQs
- What was the hidradenitis suppurativa market size & share (%) distribution in 2020, and what would it look like by 2034?
- What would be the hidradenitis suppurativa treatment market size as well as Hidradenitis Suppurativa treatment market size by therapies across the 7MM during the study period (2020–2034)?
- What are the key findings about the Hidradenitis Suppurativa treatment market across the 7MM, and which country will have the largest hidradenitis suppurativa market size during the study period (2020–2034)?
- At what CAGR, the hidradenitis suppurativa treatment market is expected to grow at the 7MM level during the study period (2020–2034)?
- What are the disease risks, burdens, and hidradenitis suppurativa unmet needs?
- What is the historical hidradenitis suppurativa patient pool in the United States, EU4 (Germany, France, Italy, and Spain) and the UK, and Japan?
- What would be the forecasted patient pool of hidradenitis suppurativa at the 7MM level?
- What will be the growth opportunities across the 7MM concerning the hidradenitis suppurativa patient population?
- Amon the 7MM, which country would have the most prevalent cases of hidradenitis suppurativa during the study period (2020–2034)?
- How many Hidradenitis Suppurativa Companies are developing therapies for the treatment of hidradenitis suppurativa?
- How many emerging therapies are in the mid-stage and late stage of development for the hidradenitis suppurativa treatment?
- What are the key collaborations (industry–industry, industry-academia), Mergers and acquisitions, and licensing activities related to hidradenitis suppurativa therapies?
- What are the recent novel therapies, targets, Hidradenitis Suppurativa mechanisms of action, and technologies developed to overcome the limitations of existing therapies?
- What are the approved biosimilars for HUMIRA?
- What are the clinical studies going on for hidradenitis suppurativa and their status?
- What are the 7MM historical and forecasted hidradenitis suppurativa treatment market?
Reasons to Buy
- The patient-based Hidradenitis Suppurativa marke forecasting report will help in developing business strategies by understanding trends shaping and driving the hidradenitis suppurativa treatment market.
- To understand the future market competition in the hidradenitis suppurativa market and an insightful review of the SWOT analysis of hidradenitis suppurativa.
- Organize sales and marketing efforts by identifying the best opportunities for hidradenitis suppurativa in the US, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
- Identification of strong upcoming players in the Hidradenitis Suppurativa treatment market will help in devising strategies that will help in getting ahead of competitors.
- Organize sales and marketing efforts by identifying the best opportunities for the hidradenitis suppurativa treatment market
- To understand the future market competition in the hidradenitis suppurativa treatment market
Explore a Wealth of Information through our Related Articles