Novartis Cosentyx: First Biologic Hidradenitis Suppurativa Treatment After Almost Ten Years
Nov 24, 2023
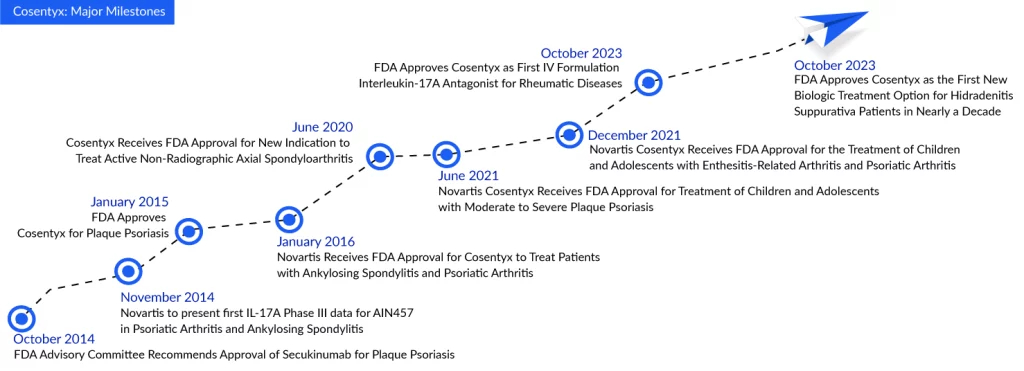
While another inflammatory biologic is making strides in the hidradenitis suppurativa treatment domain, Cosentyx by Novartis currently holds a significant lead. This advantage is crucial for Swiss Pharma, especially as dermatology experts anticipate strong competition from UCB’s bimekizumab, recently approved as Bimzelx for plaque psoriasis treatment and seeking approval for hidradenitis suppurativa treatment as well. On October 31, the FDA approved Cosentyx (secukinumab) to treat moderate to severe hidradenitis suppurativa in adults, marking the first approval of a new biologic for this skin condition in nearly a decade.
Hidradenitis Suppurativa, recognized as Acne Inversa, is a complex dermatological disorder marked by the recurring development of painful nodules and suppuration, particularly in the axillary and groin regions. The absence of a definitive biological or pathological test necessitates a diagnosis based on clinical features and the chronic nature of the condition.
As per DelveInsight estimates, the total prevalent cases of hidradenitis suppurativa in the 7MM comprised approximately 6.2 million cases in 2022 and are projected to increase during the forecast period. In the US and EU, hidradenitis suppurativa is more common in females than males, but in Japan, it is the opposite, as, in Japan, males are more commonly affected than females. The highest proportion of hidradenitis suppurativa prevalent pool was observed in the 30–39 years age group among all the countries.
Downloads
Click Here To Get the Article in PDF
According to Dr. Alexa B. Kimball, the lead investigator of the SUNSHINE and SUNRISE trials and a Professor of Dermatology at Harvard Medical School, the daily impact of hidradenitis suppurativa, along with the quest for symptom relief, can persist for years. This prolonged struggle often results in both physical and emotional scarring, which is painful and irreversible. The approval of this new treatment signifies a crucial breakthrough for numerous patients who have been grappling with limited therapeutic options.
“Hidradenitis suppurativa stands as one of the most challenging and draining skin conditions. The agony of its outbreaks can be incapacitating, hindering my ability to work or engage in social activities. It takes a toll on me both physically and emotionally, fostering feelings of anxiety, stress, and isolation,” shared Donna Atherton, EdD, Founder and Chief Mission Officer of the International Association of Hidradenitis Suppurativa Network (IAHSN). “The approval of a new treatment option instills fresh hope for myself and the entire HS community, offering the prospect of relief from the burdens imposed by this disease.”
The FDA granted approval for Cosentyx in the treatment of hidradenitis suppurativa based on comprehensive analyses derived from the most extensive Phase III program in hidradenitis suppurativa history, known as SUNSHINE and SUNRISE. This groundbreaking study revealed that a greater percentage of patients receiving Cosentyx 300 mg, either biweekly or monthly, achieved a Hidradenitis Suppurativa Clinical Response (HiSCR50) compared to those on a placebo. The approved dosage for Cosentyx in hidradenitis suppurativa is 300 mg, administered every four weeks, with the flexibility to escalate to a biweekly schedule if the patient exhibits an inadequate response.
In both the SUNSHINE and SUNRISE trials, assessing Cosentyx over 16 weeks (compared to a placebo) and a 52-week treatment period, the effectiveness of Cosentyx became evident as early as Week 2. The efficacy continued to improve through Week 16 and remained noticeable up to Week 52. The safety findings of Cosentyx in these trials for hidradenitis suppurativa were in line with its established safety profile observed in trials for plaque psoriasis, reinforcing the distinct safety profile of Cosentyx.
Victor Bultó, President of Novartis US, stated that Cosentyx delivers effective and long-lasting relief from hidradenitis suppurativa symptoms, enabling people with hidradenitis suppurativa to lead their lives with confidence. This approval for the sixth indication of Cosentyx underscores our unwavering commitment to transforming the landscape of medicine for individuals grappling with immunological diseases, with continued research in various conditions.
Novartis has pinned its hopes on Cosentyx’s approval for hidradenitis suppurativa treatment, coupled with potential expansions into giant cell arteritis and lupus nephritis, to propel the medication into its next phase of growth. If all three indications prove successful, Cosentyx has the potential to generate an additional $2 billion in peak sales, according to Novartis. In the third quarter, Cosentyx recorded approximately $1.3 billion in sales, marking a 4% increase over the $1.27 billion achieved during the same period in 2022. Cumulatively, for the initial nine months of 2023, Cosentyx has amassed a total revenue of $3.7 billion.
Despite Novartis securing approval for Cosentyx in hidradenitis suppurativa, the drug may face stiff competition from UCB’s bimekizumab, which recently presented data at the American Academy of Dermatology’s annual meeting. The data indicated that bimekizumab’s efficacy was “incrementally better than” Cosentyx, as it significantly outperformed the placebo in the proportion of patients achieving at least a 50% reduction in inflammatory lesions.
Bimekizumab by UCB Biopharma is the first humanized monoclonal IgG1 antibody that potently and selectively neutralizes both IL-17A and IL-17F, two key cytokines driving inflammatory processes. IL-17A and IL-17F are the most closely related members of the IL-17 family of cytokines. They are both co-expressed at sites of inflammation and have overlapping proinflammatory functions. Both IL-17A and IL-17F can independently cooperate with other inflammatory mediators to drive chronic inflammation and tissue destruction.
Despite encountering regulatory challenges, bimekizumab, now known as Bimzelx after securing approval for psoriasis treatment, is gearing up for further regulatory milestones. In October, the company announced plans to swiftly submit applications for additional indications in the US, marking the next step in its regulatory journey.

Downloads
Article in PDF
Recent Articles
- GSK consolidates DT and TT vaccine, AZ gears up for FDA filing, Novartis comes up with flex prici...
- Snippet : Migraine
- Merck to Acquire Harpoon Therapeutics; Novo Nordisk Enters Into Collaborations with Omega Therape...
- Mylan closing Illinois plant; Insys spends $24M; Roche’s Ocrevus; Greece’s corruption prose...
- The Business Cocktail