Leptomeningeal Metastases Market Summary
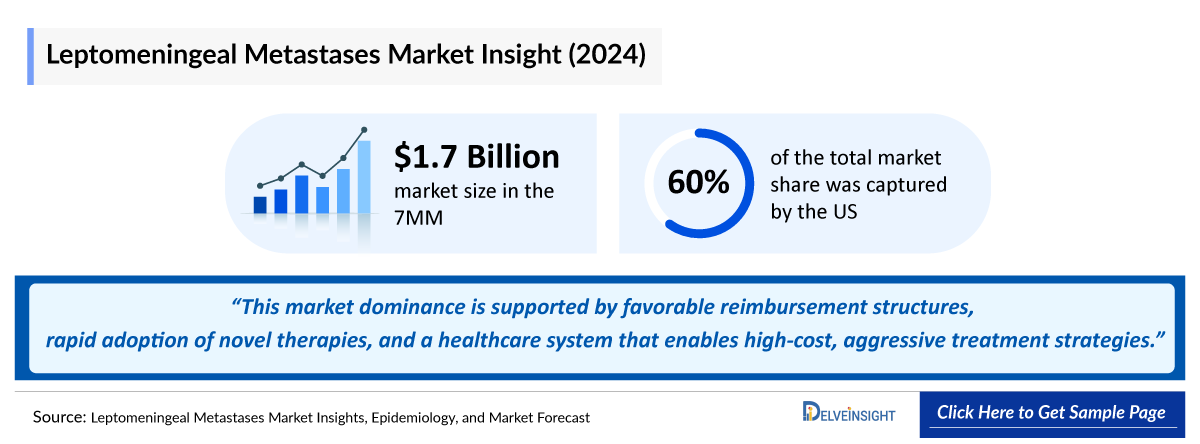
- The Leptomeningeal Metastases Market in the 7MM was valued at approximately USD 1700 million in 2024 over the forecast period from 2025 to 2034.
- The Leptomeningeal Metastases Market is projected to grow at a significant CAGR by 2034 in leading countries (US, EU4, UK, and Japan)
Leptomeningeal Metastases Market and Epidemiology Analysis
- Leptomeningeal metastases, also known as leptomeningeal carcinomatosis or neoplastic meningitis or carcinomatous meningitis or leptomeningeal disease, represent a serious and often debilitating complication of advanced metastatic solid tumors.
- Leptomeningeal metastases, frequently seen in breast, lung, or melanoma cancers, cause CSF buildup and increased brain pressure, leading to headaches, balance issues, and cranial nerve-related weakness, numbness, or pain.
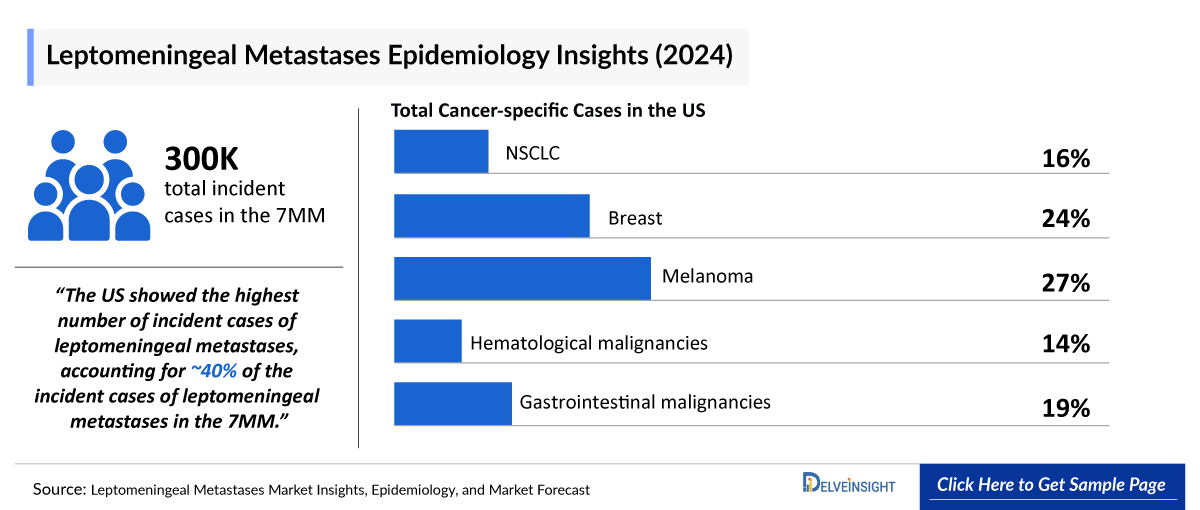
- In 2024, the Leptomeningeal Metastases Incident Cases were approximately 300,000 in the 7MM, reflecting a significant clinical burden.
- As per DelveInsight analysis, the US showed the highest number of incident cases of leptomeningeal metastases, accounting for approximately 40% of the incident cases of leptomeningeal metastases in the 7MM in 2024.
- Despite the high burden, no therapies are currently approved specifically for leptomeningeal metastases. Management remains palliative and multimodal, focusing on symptom control through a combination of off-label agents such as methotrexate, cytarabine, temozolomide, capecitabine, topotecan, and lapatinib, alongside radiation therapy. These approaches aim to slow disease progression and maintain neurological function.
- In 2024, the US captured nearly 60% of the leptomeningeal metastases market, driven by extensive use of systemic, targeted, intrathecal, and radiotherapy-based interventions. This market dominance is supported by favorable reimbursement structures, rapid adoption of novel therapies, and a healthcare system that enables high-cost, aggressive treatment strategies.
- Pharmaceutical engagement in leptomeningeal metastases has largely remained limited to supportive roles, with most companies contributing investigational agents or partial funding to academic-led trials rather than spearheading full-scale development programs. However, notable exceptions exist. Plus Therapeutics is actively developing REYOBIQ, a targeted radiotherapeutic designed specifically for leptomeningeal metastases, while Angiochem previously advanced ANG1005, a peptide-drug conjugate engineered to cross both the blood–brain and blood–CSF barriers, although its development is currently stalled.
- The emerging leptomeningeal metastases treatment landscape has also faced significant setbacks. For instance, Y-mAbs Therapeutics’ Omburtamab, a radiolabeled monoclonal antibody targeting B7-H3 in CNS tumors and LM, completed pivotal studies but received a Complete Response Letter (CRL) from the FDA in 2022. The rejection was due to insufficient clinical benefit and concerns over study design
- This development is completely unprecedented, driven not by regulatory precedent but by emerging clinical signals, marking a pivotal shift where therapeutic innovation in leptomeningeal metastases is finally being shaped by promising efficacy data rather than historical treatment paradigms.
Key Factors Driving the Growth of the Leptomeningeal Metastases Market
Rising Incidence of Central Nervous System Metastases
Advancements in systemic cancer therapies have improved overall survival rates, leading to a higher prevalence of CNS metastases, including leptomeningeal metastases. As patients live longer, the occurrence of leptomeningeal metastases as a complication has increased, thereby expanding the patient population requiring specialized treatment. In 2024, the total incident cases of leptomeningeal metastases were approximately 120K in the US, reflecting its significant disease burden and the pressing need for improved diagnostic tools and more effective treatments.
Significant Unmet Clinical Needs
Currently, there are no FDA-approved therapies specifically for leptomeningeal metastases, and the condition is often diagnosed late due to the lack of effective screening methods. This gap presents a substantial opportunity for the development of targeted treatments that can address the unique challenges of leptomeningeal metastases, such as the blood–brain barrier and limited drug penetration into the cerebrospinal fluid.
Emerging Leptomeningeal Metastases Therapies
The development of novel leptomeningeal metastases therapies is gaining momentum. For instance, Plus Therapeutics is advancing Rhenium (186Re) Obisbemeda, a radiotherapeutic targeting leptomeningeal metastases, currently undergoing clinical trials. Additionally, antibody-drug conjugates like Patritumab deruxtecan (Daiichi Sankyo) have shown promising activity in treating leptomeningeal metastases, offering hope for patients with limited options.
Leptomeningeal Metastases Market Report Summary
- The Leptomeningeal Metastases Therapeutics Market Report offers extensive knowledge regarding the epidemiology segments and predictions, presenting a deep understanding of the potential future growth in diagnosis rates, disease progression, and treatment guidelines. It provides comprehensive insights into these aspects, enabling a thorough assessment of the subject matter.
- Additionally, an all-inclusive account of the current management techniques and emerging therapies, and the elaborate profiles of prominent therapies that would impact the current Leptomeningeal Metastases Treatment Market landscape and result in an overall market shift has been provided in the report.
- The Leptomeningeal Metastases Treatment Market Report also encompasses a comprehensive analysis of the leptomeningeal metastases drugs market, providing an in-depth examination of its historical and projected market size (2020–2034). It also includes the market share of therapies, detailed assumptions, and the underlying rationale for our methodology. The report also includes drug outreach coverage in the 7MM region.
- The Leptomeningeal Metastases Treatment Market Report includes qualitative insights that provide an edge while developing business strategies by understanding trends through SWOT analysis and expert insights/KOL views, including experts from various hospitals and prominent universities, patient journey, and treatment preferences that help shape and drive the 7MM leptomeningeal metastases market.
Scope of the Leptomeningeal Metastases Market Report | |
|
Study Period |
2020–2034 |
|
Forecast Period |
2025–2034 |
|
Geographies Covered |
US, EU4 (Germany, France, Italy, and Spain) and the UK, and Japan |
|
Leptomeningeal Metastases Epidemiology |
Segmented by:
|
|
Leptomeningeal Metastases Market |
Segmented by:
|
|
Leptomeningeal Metastases Market Analysis |
|
Leptomeningeal Metastases Drug Analysis
The section dedicated to drugs in the leptomeningeal metastases treatment market report provides an in-depth evaluation of late-stage pipeline drugs related to leptomeningeal metastases. The drug chapters section provides valuable information on various aspects related to leptomeningeal metastases clinical trials, such as the pharmacological mechanisms of the drugs involved, designations, approval status, patent information, and a comprehensive analysis of the pros and cons associated with each drug. Furthermore, it presents the most recent news updates and press releases on drugs targeting leptomeningeal metastases.
Leptomeningeal Metastases Emerging Therapies
-
REYOBIQ (rhenium Re186 obisbemeda): Plus Therapeutics
REYOBIQ (rhenium Re186 obisbemeda) is an innovative injectable radiotherapy designed to deliver high-dose, targeted radiation directly to central nervous system tumors with a focus on safety, efficacy, and ease of administration. Leveraging the unique properties of rhenium-186, its short half-life, beta emissions for tumor destruction, and gamma emissions for real-time imaging REYOBIQ offers a promising approach for CNS therapies. It is currently under evaluation for the Leptomeningeal Metastases Treatment in the ongoing ReSPECT-LM clinical trials.
-
- In July 2025 Plus Therapeutics stated that it will unveil findings from its ReSPECT-LM clinical trial and lead a sponsored educational symposium during the upcoming SNO/ASCO CNS Metastases Conference, taking place from August 14 to 16, 2025.
- In March 2025, Plus Therapeutics reported that the US FDA has granted Orphan Drug Designation (ODD) to REYOBIQ (Rhenium (186Re) Obisbemeda) for the Leptomeningeal Metastases Treatment in patients with lung cancer.
-
Paxalisib: Kazia Therapeutics/Genentech
Paxalisib (GDC-0084) is an experimental therapy under development for treating a range of brain cancers. It acts as a brain-penetrant inhibitor of the PI3K/Akt/mTOR signaling pathway, which plays a vital role in regulating cell growth and division. What sets paxalisib apart from other agents in its class is its design tailored to cross the blood-brain barrier enabling efficient drug delivery to brain tissue, a critical advantage in targeting central nervous system malignancies.
-
- In October 2024, at the 66th Annual Meeting of ASTRO, Kazia Therapeutics shared Phase I data revealing that a 45 mg dose of paxalisib, when combined with radiotherapy, resulted in a 67% partial response rate in patients with brain or leptomeningeal metastases harboring PI3K mutations. Notably, more than two-thirds of patients treated at the maximum tolerated dose (MTD) showed intracranial responses—exceeding historical benchmarks for radiation therapy alone.
- In February 2024, Kazia Therapeutics reported the early conclusion of a clinical trial after successfully meeting its primary endpoint. The combination of paxalisib and radiotherapy demonstrated encouraging potential for treating brain metastases associated with PI3K pathway mutations.
|
Product |
Company |
Indication |
Phase |
Molecule Type |
MOA |
ROA |
|
Rhenium-186 obisbemeda (REYOBIQ)1 |
Plus Therapeutics |
Leptomeningeal metastases |
I |
Radiopharmaceutical |
Ionising radiation emitters |
Intraventricular |
|
HSV G2072 |
M.D. Anderson Cancer Center (Collaborator: Treovir) |
Recurrent or Refractory Cerebellar Brain Tumors |
I |
Oncolytic virus |
Ribonucleotide reductase inhibitors |
IV Infusion |
|
AZD1390 Given With Radiation Therapy3 |
AstraZeneca |
Brain Cancer |
I |
Small molecule |
Ataxia telangiectasia mutated protein inhibitors |
Oral |
|
All regimens are drawn from interventional studies explicitly mentioning leptomeningeal metastases (& its synonym). ∙ Studies were included only if conducted by industry or by individual investigators, institutes, or research centers in collaboration with industry sponsors. ∙ Trials were selected irrespective of whether results are currently available or whether the study is listed in the sponsoring company’s pipeline for the indication. ∙ While most studies primarily focused on brain cancer, they included LMD patients in their inclusion criteria, thus the studies got chosen | ||||||
Leptomeningeal Metastases Market Outlook
During the forecast period (2025–2034), pipeline candidates such as Plus Therapeutics’ REYOBIQ (rhenium Re186 obisbemeda), Kazia Therapeutics/Genentech Paxalisib, and others are expected to drive the rise in leptomeningeal metastases market size.
- In 2024, the Leptomeningeal Metastases Market Size in 7MM was approximately USD 1700 million, which is expected to rise in 2034.
- In 2024, in the EU4 and the UK, Germany has the highest Leptomeningeal Metastases Market Size at approximately USD 160 million, followed by the UK with approximately USD 130 million.
Leptomeningeal Metastases Disease Understanding
Leptomeningeal Metastases Overview
Leptomeningeal metastases, also known as leptomeningeal carcinomatosis or neoplastic meningitis, refer to the dissemination of malignant cells into the leptomeninges (the pia and arachnoid mater) surrounding the brain and spinal cord, as well as into the CSF. These cells disrupt normal CSF drainage pathways, resulting in fluid buildup and increased intracranial pressure. The ensuing pressure causes a range of nonspecific yet troubling symptoms, often more prominent in the morning, including headaches, nausea, vision problems, and impaired balance or coordination. Cranial nerve involvement can further cause numbness, muscle weakness, or pain. This condition typically stems from the progression of systemic cancers, most frequently those originating in the breast, lungs, gastrointestinal tract, hematologic system, melanoma, or the CNS.
Several risk factors are linked to the development of leptomeningeal metastases. In brain tumors, leptomeningeal metastases are more likely after surgical resection of parenchymal metastases especially in the posterior fossa—when intraventricular access or piecemeal removal is involved. In breast cancer, lobular histology, HER2-positive, and triple-negative subtypes carry a higher risk. In Non-small Cell Lung Cancer (NSCLC), EGFR mutations and ALK translocations are associated with increased leptomeningeal metastases Incidence
Leptomeningeal Metastases Diagnosis
Diagnosing leptomeningeal metastases begins with a comprehensive review of the patient’s medical history and a detailed physical and neurological examination. This assessment helps determine the extent of neurological involvement and raises suspicion for leptomeningeal disease. Once suspected, CSF analysis and neuroimaging become essential next steps. Tumor markers in CSF are valuable for early detection, monitoring treatment response, and predicting outcomes. General markers like lactate dehydrogenase and beta-2 microglobulin, along with specific markers such as alpha-fetoprotein (AFP), beta-human chorionic gonadotropin (ß-hCG), and carcinoembryonic antigen (CEA), can provide important diagnostic insights.
Leptomeningeal Metastases Treatment
The management of leptomeningeal metastases prioritizes symptom relief and disease control through a coordinated, multidisciplinary approach. Due to the diffuse nature of the condition, surgical excision is not a viable option. Treatment usually begins with targeted radiation therapy to alleviate neurological symptoms, followed by systemic therapies capable of penetrating the central nervous system. In selected patients presenting with obstructive hydrocephalus, ventriculoperitoneal shunting (VPS) may provide effective palliation, with over 80% reporting symptomatic improvement and a relatively low rate of complications. Recent studies also indicate a potential survival benefit when VPS is combined with intrathecal therapy. A comprehensive treatment plan often integrates various modalities, including radiation therapy, conventional systemic therapies, intrathecal therapy, immunotherapy, and molecularly targeted treatments, each tailored to address the unique needs of the patient and the biology of the disease.
Leptomeningeal Metastases Epidemiology
The leptomeningeal metastases epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by Total Incident Cases of Leptomeningeal Metastases, Incident Cases of Leptomeningeal Metastases by Cancer Type, Leptomeningeal Metastases by risk type, Gender-specific Cases of Leptomeningeal Metastases, Leptomeningeal Metastases Treated Patient Pool in the United States, EU4 countries (Germany, France, Italy, Spain), the United Kingdom, and Japan from 2020 to 2034.
Key Findings from the Leptomeningeal Metastases Epidemiological Analysis
- In 2024, the total Leptomeningeal Metastases Incident Cases were approximately 120,000 in the US, reflecting its significant disease burden and the pressing need for improved diagnostic tools and more effective treatments.
- In 2024, breast cancer recorded the highest incident cases of leptomeningeal metastases among all cancer types across EU4 and the UK, with approximately 30,000 cases reported.
- In Germany in 2024, incident leptomeningeal metastases cases were stratified by risk classification, with 66% of patients falling under the “good risk” category and the remaining 34% categorized as “poor risk.” This distribution highlights a predominance of favorable prognostic profiles within the affected population.
Leptomeningeal Metastases Epidemiology Segmentation
- Total Leptomeningeal Metastases Incident Cases
- Leptomeningeal Metastases Incident Cases by Cancer Type
- Leptomeningeal Metastases by risk type
- Leptomeningeal Metastases Gender-Specific Cases
- Leptomeningeal Metastases Treated Patient Pool
Latest KOL Views on Leptomeningeal Metastases
To stay abreast of the latest trends in the market, we conduct primary research by seeking the opinions of Key Opinion Leaders (KOLs) and Subject Matter Experts (SMEs) who work in the relevant field. This helps us fill any gaps in data and validate our secondary research. We have reached out to industry experts to gather insights on various aspects of leptomeningeal metastases, including the evolving treatment landscape, patients’ reliance on conventional therapies, their acceptance of therapy switching, drug uptake, and challenges related to accessibility. The experts we contacted included medical/scientific writers, professors, and researchers from prestigious universities in the US, Europe, the UK, and Japan.
Our team of analysts at DelveInsight connected with more than 10 KOLs across the 7MM. We contacted institutions such as the University of Colorado Cancer Center, the Georgetown University Hospital, Department of Medical Oncology, and South Miyagi Medical Center, etc., among others. By obtaining the opinions of these experts, we gained a better understanding of the current and emerging treatment patterns in the leptomeningeal metastases market, which will assist our clients in analyzing the overall epidemiology and market scenario.
Leptomeningeal Metastases Report Qualitative Analysis
We perform Qualitative and Market Intelligence analysis using various approaches, such as SWOT analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the Analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving treatment landscape.
Leptomeningeal Metastases Market Access and Reimbursement
Reimbursement plays a critical role in how innovative treatments can enter the market. The cost of the medicine, compared to the benefit it provides to patients who are being treated, sometimes determines whether or not it will be reimbursed. Regulatory status, target population size, the setting of treatment, unmet needs, the number of incremental benefit claims, and prices can all affect market access and reimbursement possibilities.
Leptomeningeal Metastases Market Report Insights
- Patient-based Leptomeningeal Metastases Market Forecasting
- Therapeutic Approaches
- Leptomeningeal Metastases Market Size and Trends
- Existing Leptomeningeal Metastases Drugs Market Opportunity
Leptomeningeal Metastases Market Report Key Strengths
- 10-year Leptomeningeal Metastases Market Forecast
- The 7MM Coverage
- Leptomeningeal Metastases Epidemiology Segmentation
- Key Cross Competition
Leptomeningeal Metastases Market Report Assessment
- Current Leptomeningeal Metastases Treatment Practices
- Reimbursements
- Leptomeningeal Metastases Drugs Market Attractiveness
- Leptomeningeal Metastases Qualitative Analysis (SWOT, Unmet Needs)
Key Questions Answered in the Leptomeningeal Metastases Report
Leptomeningeal Metastases Market Insights
- Would there be any changes observed in the current treatment approach?
- Will there be any improvements in leptomeningeal metastases management recommendations?
- Would research and development advances pave the way for future tests and therapies for leptomeningeal metastases?
- Would the diagnostic testing space experience a significant impact and lead to a positive shift in the treatment landscape of leptomeningeal metastases?
- What kind of uptake will the new therapies witness in the coming years in leptomeningeal metastases patients?
Stay updated with us for Recent Articles