Mitochondrial Myopathies Market Summary
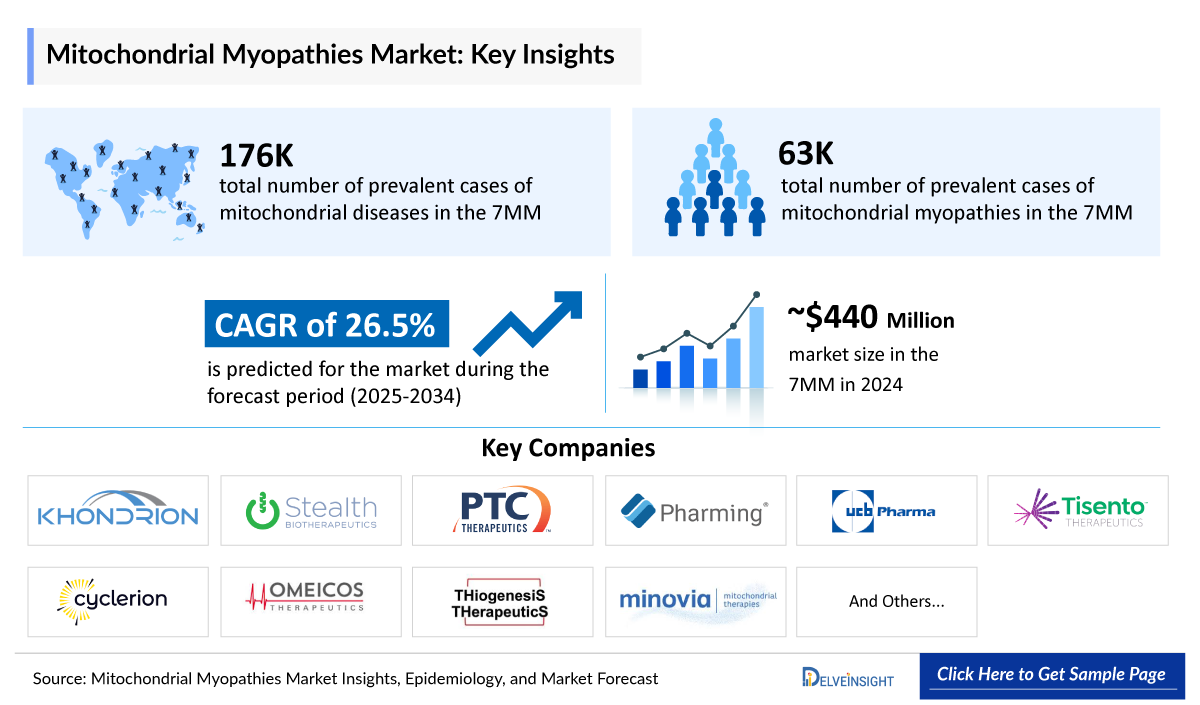
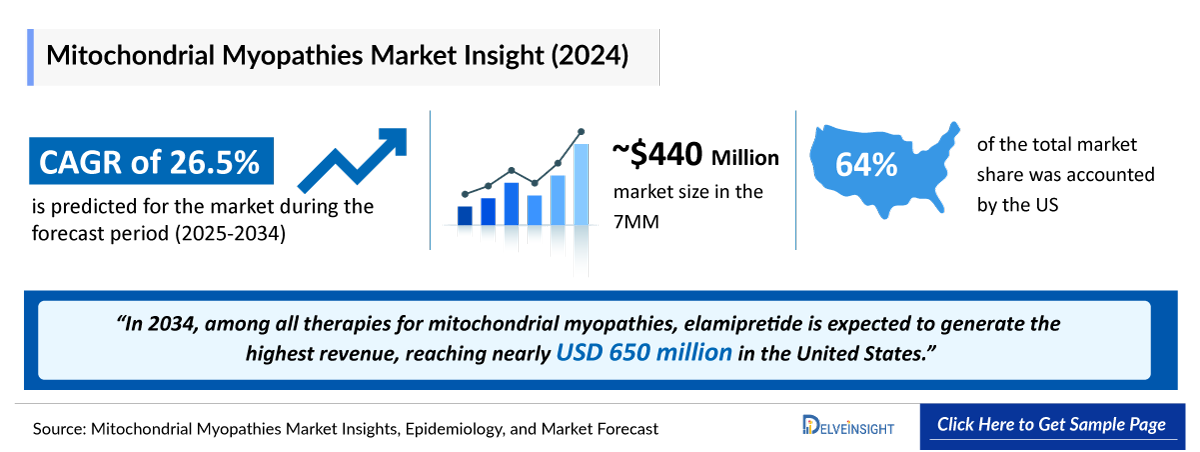
- The Mitochondrial Myopathies Market in the 7MM was valued at approximately USD ~440 million in 2025, over the forecast period from 2025 to 2034.
- The Mitochondrial Myopathies Market is projected to grow at a CAGR of 26.5% by 2034 in the leading countries (US, EU4, UK, and Japan).
 Mitochondrial Myopathies Market and Epidemiology Analysis
Mitochondrial Myopathies Market and Epidemiology Analysis
- Mitochondrial diseases have a reported prevalence 8–9 times higher in Western datasets compared to Japan’s estimates based on the National Database (NDB). This difference is unlikely to reflect biological factors and more likely results from systematic under-detection in Japan, attributed to lower clinical suspicion, fewer specialist centers per capita, and historically limited access to genetic testing.
- Mitochondrial myopathies represent a broad and diverse group of genetic disorders, exhibiting a wide range of phenotypic manifestations. They are part of the superfamily of mitochondrial diseases, which is derived from a primary dysfunction of the mitochondrial respiratory chain, leading mostly to muscle disorders, hence the term mitochondrial myopathies.
- Universal newborn screening for select mitochondrial mutations is limited to pilot programs in parts of the US, whereas other countries lack standardized inclusion, contributing to geography-specific case detection gaps.
- The main mitochondrial diseases (including myopathies) include Chronic Progressive External Ophthalmoplegia (CPEO), Primary Coenzyme Q10 Deficiency, Mitochondrial Encephalomyopathy, Lactic Acidosis, and Stroke-like episodes (MELAS), Myoclonic Epilepsy with Ragged-Red Fibers (MERRF), Kearns-Sayre Syndrome (KSS), Thymidine Kinase 2 Deficiency (TK2d), Barth Syndrome, Pyruvate Dehydrogenase Complex Deficiency (PDCD), and Leigh Syndrome.
- Molecular diagnostic tools such as Next-generation Sequencing (NGS) and Polymerase Chain Reaction (PCR) now play a crucial role in identifying the genetic basis of mitochondrial myopathies, enabling earlier and more accurate diagnoses.
- There are currently no approved Disease-modifying Therapies (DMTs) for mitochondrial myopathies; treatment remains supportive and symptom-based. Management typically includes mitochondrial supplements (e.g., Coenzyme Q10 (CoQ10, L-carnitine, and vitamins), symptom-specific medications, tailored dietary strategies, and physical or occupational therapy. While these approaches aim to optimize function and quality of life, evidence for their efficacy remains limited.
- The “mitochondrial cocktail”, a combination of supplements believed to support mitochondrial function, is used based on limited evidence (except for CoQ10), and while often introduced sequentially to assess benefit due to cost and lack of coverage, it may be initiated all at once in severe cases.
- Despite growing interest in mitochondrial disorders, no clinical trials are directly targeting mitochondrial myopathies, with current research focusing on broader or overlapping conditions like MELAS and PMD.
- The current Mitochondrial Diseases Pipeline is scarce, which is featuring diverse mechanisms of action across oral small molecules like Sonlicromanol (Khondrion; redox modulator), KL1333 (Pharming; NAD?/NADH modulation), Zagociguat (Tisento/Cyclerion; sGC stimulation), TTI-0102 (Thiogenesis; thiol-based mitochondrial support), and MT1621 (UCB; mtDNA replication fidelity restoration), alongside Elamipretide (Stealth BioTherapeutics; SC peptide targeting cardiolipin stabilization), and others.
- Additionally, Mitochondrial Myopathies Drugs in development include Vatiquinone (EPI-743) by PTC Therapeutics, OMT-282 by Omeicos Therapeutics GmbH, TTI-0102 by Thiogenesis Therapeutics, MNV-2014 by Minovia Therapeutics, BPM31510 (IV) by BPGbio, PX5786 by Pretzel Therapeutics, and others.
- Development of mitochondrial myopathy therapies has seen setbacks, with Reneo’s mavodelpar (REN001) failing its Phase III trial and being withdrawn from the EU orphan registry in 2024. Post-merger with OnKure, it’s no longer in the pipeline. Similarly, Astellas Pharmaceuticals discontinued ASP0367 after Phase II, highlighting challenges in achieving clinical efficacy in this area.
- Patients with mitochondrial myopathies face numerous challenges, including delayed diagnosis or misdiagnosis due to limited awareness among healthcare providers and patients, as well as a highly variable and unpredictable disease course, complicating timely and effective treatment initiation.
Mitochondrial Myopathies Market Size and Forecast
- 2025 Mitochondrial Myopathies Market Size: USD 440 Million
- 2034 Projected Mitochondrial Myopathies Market Size: USD 3,645 million
- Mitochondrial Myopathies Growth Rate (2025-2034): 26.50% CAGR
- Largest Mitochondrial Myopathies Market: United States
Request for unlocking the Sample Page of the "Mitochondrial Myopathies Market"
Factors Impacting the Mitochondrial Myopathies Market Growth
-
Improved diagnostics and expanding genetic testing
The wider availability of next-generation sequencing, the broader use of whole-exome/genome testing, and enhanced clinical awareness are increasing diagnosis rates and enabling patient identification for trials and targeted treatments. The growth of the rare-disease genetic testing market is a major driver.
-
Increasing investment, M&A, and industry interest
Growing recognition of mitochondria as therapeutic targets (across neurology, metabolic, and cardiac indications) has driven funding rounds, start-ups focused on mitochondrial biology, and strategic partnerships, increasing the capital available to carry therapies through clinical trials and commercialization.
-
Expanding therapeutic mitochondrial myopathies clinical trial activity
The current pipeline for mitochondrial diseases is scarce, featuring diverse mechanisms of action across oral small molecules like Sonlicromanol (Khondrion; redox modulator), KL1333 (Pharming; NAD+/NADH modulation), Zagociguat (Tisento/Cyclerion; sGC stimulation), TTI-0102 (Thiogenesis; thiol-based mitochondrial support), and MT1621 (UCB; mtDNA replication fidelity restoration), alongside Elamipretide (Stealth BioTherapeutics; SC peptide targeting cardiolipin stabilization), and others. Additionally, other drugs in development include Vatiquinone (EPI-743) by PTC Therapeutics, OMT-282 by Omeicos Therapeutics GmbH, TTI-0102 by Thiogenesis Therapeutics, MNV-2014 by Minovia Therapeutics, BPM31510 (IV) by BPGbio, PX5786 by Pretzel Therapeutics, and others.
-
Lack of approved disease-modifying mitochondrial myopathies therapies creates opportunities
Mitochondrial myopathies remain largely managed with symptomatic care and “mito-cocktails.” The absence of approved DMTs creates both clinical urgency and commercial opportunity for therapies that can alter the disease course.
DelveInsight's ‘Mitochondrial Myopathies Treatment Market Insights, Epidemiology and Market Forecast – 2034’ report delivers an in-depth understanding of the mitochondrial myopathies, historical and forecasted epidemiology, as well as the mitochondrial myopathies market trends in the United States, EU4 (Germany, Spain, Italy, and France) and the United Kingdom, and Japan.
The Mitochondrial Myopathies Treatment Market Report provides current treatment practices, emerging drugs, market share of individual therapies, and current and forecasted 7MM mitochondrial myopathies market size from 2020 to 2034. The report also covers current mitochondrial myopathies treatment practices/algorithms and unmet medical needs to curate the best opportunities and assess the market’s underlying potential.
Scope of the Mitochondrial Myopathies Market Report | |
|
Study Period |
2020–2034 |
|
Forecast Period |
2025–2034 |
|
Geographies Covered |
|
|
Mitochondrial Myopathies Epidemiology |
Segmented By
|
|
Mitochondrial Myopathies Companies |
|
|
Mitochondrial Myopathies Therapies |
|
|
Mitochondrial Myopathies Market |
Segmented by
|
|
Analysis |
|
Mitochondrial Myopathies Disease Understanding
Mitochondrial Myopathies Overview
Mitochondrial myopathies are progressive muscle disorders primarily resulting from impaired oxidative phosphorylation (OXPHOS) within the mitochondria. This dysfunction leads to reduced ATP production, especially affecting skeletal muscle, where energy demand is high. Mitochondria contain their genetic material, mitochondrial DNA (mtDNA). However, the mitochondrial function is also under the control of the nuclear or autosomal DNA, i.e, nDNA, which regulates the maintenance of mtDNA, the mitochondrial protein synthesis, and the synthesis and function of the respiratory chain complexes and cofactors.
Mitochondrial myopathies can be triggered or worsened by factors such as genetic mutations, aging, infections, lack of physical activity, obesity, or as a secondary symptom of other underlying conditions. Mitochondrial myopathies present with a wide range of symptoms, commonly including muscle weakness, early fatigue, and exertional intolerance, which significantly impact daily functioning. Additional manifestations may involve ptosis (drooping eyelids), seizures, and stroke-like episodes, reflecting the disorder’s effect on multiple systems. In more severe cases, patients may also experience stunted growth and liver failure, underscoring the progressive and multisystemic nature of the disease. Mitochondrial myopathies include diverse syndromes like Mitochondrial Encephalomyopathy, Lactic Acidosis, and Stroke-like episodes (MELAS), Myoclonus Epilepsy with Ragged Red Fibers (MERRF), Leigh syndrome, Kearns-Sayre Syndrome (KSS), and others, each with distinct genetic causes.
Mitochondrial Myopathies Diagnosis
Diagnosing mitochondrial myopathies requires a systematic, individualized approach combining clinical, biochemical, genetic, and histological assessments. Initial evaluations include symptom-based testing such as CK, lactate levels, EMG, MRI, cardiac and endocrine evaluations, and CNS imaging if relevant. These help identify affected organ systems but are not disease-specific. Genetic testing using next-generation sequencing (NGS) is the preferred diagnostic tool, capable of detecting mtDNA and nDNA mutations. While it reduces the need for biopsies, variants of unknown significance (VUS) may require further testing, including muscle biopsy. Muscle biopsy remains valuable, especially when blood or buccal samples are inconclusive, as it can reveal ragged-red fibers, COX-negative fibers, and mtDNA deletions more readily. EMG may assist in excluding mimics, while exercise testing (e.g., cycle ergometry, aerobic forearm test) can assess mitochondrial function and help when genetic results are ambiguous.
Further details are provided in the report.
Mitochondrial Myopathies Treatment
Management of mitochondrial myopathy focuses on symptom relief, slowing disease progression, and addressing comorbidities through a multidisciplinary approach. This includes care from physiotherapists, speech and occupational therapists, dietitians, and medical specialists. Genetic counseling and regular systemic screenings are essential post-diagnosis. Treatment options often involve dietary supplements (“mitococktails” with CoQ10, B vitamins, etc.), although evidence of efficacy remains limited. Exercise therapy is a key intervention, shown to enhance mitochondrial function and reduce mutated mtDNA levels. Preventive care, including vaccinations and avoidance of mitochondrial-toxic drugs (e.g., valproic acid, metformin), is crucial. Follow-ups every 6–12 months, tailored to disease severity, are recommended.
Mitochondrial Myopathies Epidemiology
As the market is derived using a patient-based model, the mitochondrial myopathies epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented total prevalent cases of mitochondrial diseases, total prevalent cases of mitochondrial myopathies, total prevalent cases of specific types of mitochondrial diseases (including myopathies), mutation-specific prevalent cases of mitochondrial myopathies, age-specific prevalent cases of mitochondrial myopathies, total treated cases of mitochondrial diseases (including myopathies), total treated cases of mitochondrial myopathies in the 7MM covering the United States, EU4 (Germany, France, Italy, and Spain), United Kingdom, and Japan from 2020 to 2034.
Mitochondrial Myopathies Epidemiological Analysis and Forecast
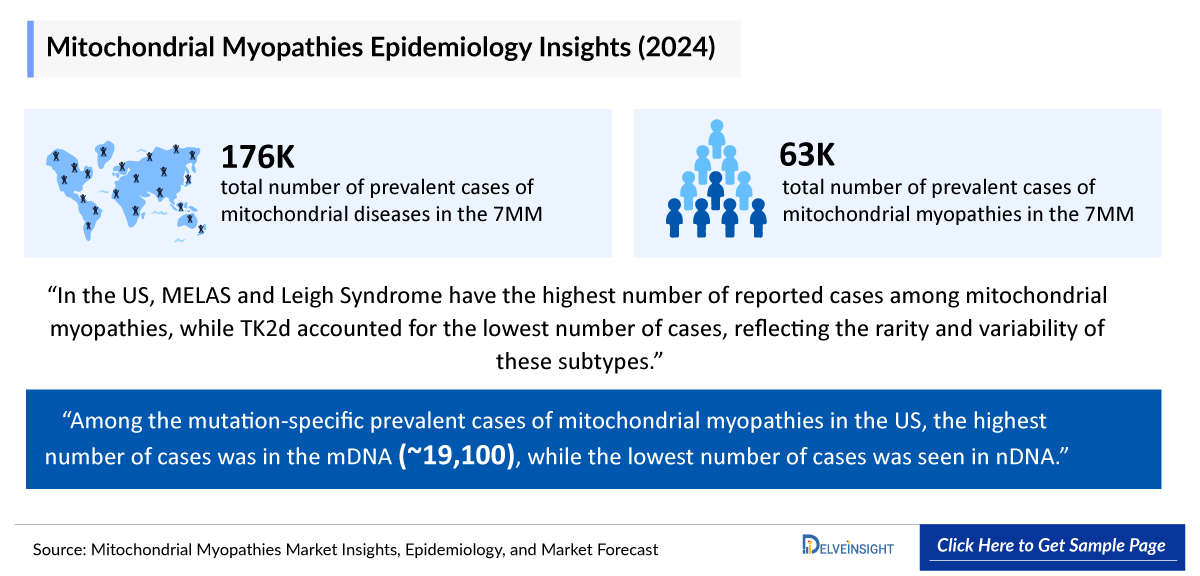
- The total number of prevalent cases of mitochondrial diseases in the 7MM ranges from ~176,400 in 2024.
- The total number of prevalent cases of mitochondrial myopathies in the 7MM ranges from ~63,200 in 2024.
- The total number of treated cases of mitochondrial myopathies in Japan is projected to reach ~3,000 in 2024.
- The US contributed to the largest Mitochondrial Myopathies Prevalent Population, accounting for ~50% of the 7MM in 2024. Whereas EU4 and the UK, and Japan accounted for the remaining total population share in 2024.
- According to DelveInsight estimates, in 2024, among the Mitochondrial Myopathies Mutation-specific Prevalent Cases in the US, the highest number of cases was in the mDNA (~19,100), while the lowest number of cases was seen in the nDNA.
- In Japan, the Mitochondrial Myopathies Age-specific prevalent cases were more in = the 18-year age group (~2,800) than in>18 age group in 2024.
- In the US, MELAS and Leigh Syndrome have the highest number of reported cases among mitochondrial myopathies, while TK2d accounted for the lowest number of cases, reflecting the rarity and variability of these subtypes.
Mitochondrial Myopathies Epidemiology Segmentation
- Total Mitochondrial Myopathies Prevalent Cases
- Total Prevalent Cases of Specific Types of Mitochondrial Diseases (Including Myopathies)
- Mitochondrial Myopathies Mutation-specific Prevalent Cases
- Mitochondrial Myopathies Age-specific Prevalent Cases
- Total Mitochondrial Myopathies Treated Cases
Mitochondrial Myopathies Drug Analysis
The drug chapter segment of the Mitochondrial Myopathies Therapeutics Market Report encloses a detailed analysis of late-stage (Phase III, Phase II, and Phase I) Mitochondrial Myopathies Pipeline Drugs. Currently, there are no approved therapies specifically indicated for mitochondrial myopathies, highlighting a significant unmet medical need. It also helps understand the mitochondrial myopathies clinical trial details, expressive pharmacological action, agreements and collaborations, approval and patent details, advantages and disadvantages of each included drug, and the latest Mitochondrial Myopathies news and press releases.
Mitochondrial Myopathies Emerging Drugs
-
Sonlicromanol (KH176): Khondrion
Sonlicromanol (formerly known as KH176) is a potential first-in-class medicine and one of the most advanced disease-modifying drug candidates for PMD is taken as a twice-a-day oral tablet in clinical development. It targets the key underlying mechanisms of mitochondrial disease based on its scientifically validated and unique triple mode of action. It is a reductive and oxidative distress modulator with anti-inflammatory properties. It has been studied in a Phase I study in healthy volunteers, in three studies (KHENERGY, KHENERGYZE, and KHENEREXT) in participants with m.3243A>G PMD, and in one study (KHENERGYC) in children with PMD.
-
Doxecitine and doxribtimine (MT1621): UCB Biosciences
Doxecitine and doxribtimine (MT1621) is a fixed-dose combination therapy that targets the underlying pathophysiology of TK2d by restoring mtDNA replication fidelity. Doxecitine and doxribtimine consist of a combination of deoxynucleosides (the building blocks of mtDNA) given orally. Deoxynucleoside combination therapy improves nucleotide balance, increases mtDNA copy number, improves cell function, and prolongs life in preclinical models of TK2d. By increasing the levels of thymidine and deoxycytidine in the body, the medicine is expected to make up for the deficiencies in TK2 activity, thereby improving the production of mitochondrial DNA and helping relieve the patient’s symptoms.
Doxecitine and doxribtimine are in clinical development for the treatment of TK2d. In the pivotal Phase II trial (NCT03845712), doxecitine and doxribtimine are administered orally up to a maximum of 800 mg/kg/day (400 mg/kg/day of dC and 400 mg/kg/day of dT) as tolerated. A Phase III (NCT04581733) was initiated in September 2022 by Zogenix; however, due to the sponsor’s decision, the trial was withdrawn.
According to the full year report 2024 of UCB, at the end of 2024, regulatory submissions of doxecitine and doxribtimine in TK2d occurred and were accepted in February 2025 for review by the European and US authorities. In the US, the application has been granted a priority review and the feedback is anticipated in the end of 2025.
-
MTP-131 (elamipretide): Stealth BioTherapeutics
Elamipretide, is a peptide compound that readily penetrates cell membranes and targets the inner mitochondrial membrane, where it binds reversibly to cardiolipin. In preclinical or clinical studies, Stealth BioTherapeutics observed that elamipretide increases mitochondrial respiration, improves electron transport chain function and ATP production, and reduces the formation of pathogenic ROS levels. This elamipretide-cardiolipin association has been shown to normalize the structure of the inner mitochondrial membrane, thereby improving mitochondrial function. Functional benefit is achieved through improvement of ATP production and interruption and potential reversal of damaging oxidative stress.
A Phase III trial (NCT03323749 [MMPOWER-3]) was terminated in February 2020 because the Part 1, double blind portion of the trial did not meet the primary endpoints, and a registrational Phase II trial (NCT02976038) was terminated in March 2020 because it also did not meet the primary endpoints.
In May 2025, the US FDA declined the current approval of elamipretide for Barth syndrome despite advisory panel’s support, offering instead a limited accelerated approval path that excludes the sickest patients.
Comparison of Emerging Drugs Under Development | ||||||
|
Drug Name |
Company |
Highest Phase |
Indication |
RoA |
MoA |
Molecule Type |
|
Sonlicromanol (KH176) |
Khondrion |
III |
PMD |
Oral |
Redox-modulator (microsomal prostaglandin E-synthase 1 (mPGES-1) inhibition) and as an antioxidant |
Small molecule |
|
Elamipretide |
Stealth BioTherapeutics |
III; II/III |
nPMD; Barth Syndrome |
SC |
Binds cardiolipin to help stabilize the mitochondrial cristae, reduce oxidative stress, and enhance ATP production |
Peptide |
|
SL-1009 |
Saol Therapeutics |
III |
PDCD |
Oral |
Inhibiting PDKs (Pyruvate Dehydrogenase Kinases) |
Small molecule |
|
Doxecitine and doxribtimine (MT1621) |
UCB |
II, filed |
TK2d |
Oral |
Restores mtDNA replication fidelity |
Small molecule |
|
KL1333 |
Pharming Technologies |
II |
mtDNA PMD (FALCON). MELAS-MIDD and KSS-CPEO spectrum disorders as well as MERRF syndrome. |
Oral |
NAD⁺/NADH modulation |
Small molecule |
|
Zagociguat |
Tisento Therapeutics and Cyclerion Therapeutics |
II |
MELAS |
Oral |
sGC stimulation |
Small molecule |
|
TTI-0102 |
Thiogenesis Therapeutics |
II |
MELAS, Leigh Syndrome Spectrum |
Oral |
Acts as a precursor to glutathione, metabolizes into taurine, and boosts CoA production to support mitochondrial function |
Small molecule |
Mitochondrial Myopathies Drug Class Insights
sGC stimulation plays a promising role in mitochondrial myopathy by targeting the nitric oxide (NO)-sGC-cGMP signaling pathway, which is often impaired in these patients. Activation of sGC increases cyclic guanosine monophosphate (cGMP) levels, leading to improved mitochondrial biogenesis, enhanced muscle perfusion, and reduced oxidative stress. This mechanism can help restore cellular energy balance, improve muscle function, and slow disease progression in mitochondrial disorders like MELAS. Agents like Zagociguat exemplify this targeted approach, showing CNS penetration and potential benefit in mitochondrial-related CNS and muscle dysfunction.
NAD?/NADH modulation plays a key role in managing mitochondrial myopathies by targeting cellular redox balance and enhancing mitochondrial function. NAD? is essential for oxidative phosphorylation, ATP production, and the activity of sirtuins, which regulate mitochondrial biogenesis and cellular repair mechanisms. Modulating the NAD?/NADH ratio helps restore energy metabolism, reduce oxidative stress, and may slow disease progression.
Mitochondrial Myopathies Market Outlook
Despite growing understanding, no approved DMTs currently exist for mitochondrial myopathies. Management remains symptom-focused and relies on individualized, multidisciplinary care, involving neurologists, dietitians, physiotherapists, and other specialists. Mitococktails—combinations of supplements like CoQ10, B vitamins, and alpha-lipoic acid—are widely used despite limited clinical evidence. Exercise and specific dietary interventions (e.g., ketogenic diet) may support mitochondrial health. Off-label use of agents like idebenone and L-arginine is increasing, though their benefits remain debated.
Prescription trends reflect empirical practices with growing emphasis on supportive and personalized care. The therapeutic landscape for mitochondrial myopathies is rapidly evolving, with several small-molecule and mitochondrial-targeting therapies advancing through clinical development. Key drugs in the pipeline include Doxecitine and Doxribtimine (UCB Biosciences), Sonlicromanol (Khondrion), KL1333 (Pharming Group), Zagociguat (Tisento Therapeutics and Cyclerion Therapeutics), TTI-0102 (Thiogenesis Therapeutics), and Elamipretide (Stealth BioTherapeutics).
The Mitochondrial Myopathies Treatment Market is growing steadily, driven by the increasing use of biologics, improved diagnosis, and rising awareness. The US accounts for the largest market size of mitochondrial myopathies, in comparison to EU4 and the UK (Germany, France, Italy, the UK, and Spain) and Japan.
- The Mitochondrial Myopathies Treatment Market Size in the 7MM was approximately USD ~440 Million in 2025, and is expected to grow at a CAGR of 26.50% over the forecast period of 2025-2034.
- Among EU4 and the UK, Germany accounted for the highest Mitochondrial Myopathies Market Size in 2024, while Spain occupied the lowest.
- In 2034, among all the therapies for mitochondrial myopathies, the highest revenue will be generated by elamipretide, i.e., nearly USD 650 million in the United States.
- The first drug is set to launch in 2026 for TK2D, after which we will observe variations in market share.
Mitochondrial Myopathies Key Updates
- Based on Pharming Technologies’ financial results for Q2 and the first half of 2025, the company expects the FALCON trial results in 2027, with potential FDA approval anticipated by the end of that year.
- In March 2025, UCB presented positive data at the MDA 2025 Conference from studies on its investigational pyrimidine nucleoside therapies, doxecitine and doxribtimine, in individuals with TK2d, showing that treatment significantly reduced mortality and improved survival in those whose symptoms began at age 12 or younger.
- In June 2025, Thiogenesis Therapeutics announced that it has received IND clearance in the US for the treatment of LSS with TTI-0102 and approval to initiate a Phase IIa clinical trial for LSS in H2 2025.
- In June 2025, Tisento Therapeutics announced that the US FDA had granted Fast track designation to zagociguat for the treatment of MELAS.
- As per the May 2025 press release of Khondrion, the Phase III trial is expected to launch in the second half of 2025 for sonlicromanol (KH176).
Mitochondrial Myopathies Drug Uptake
This section focuses on the uptake rate of potential Mitochondrial Myopathies drugs expected to be launched in the market during 2020–2034. The analysis covers the mitochondrial myopathies market's uptake by drugs, patient uptake by therapy, and sales of each drug. This section focuses on the uptake rate of potential drugs expected to be launched in the market during 2020–2034. The landscape of mitochondrial myopathies treatment has experienced a profound transformation with the uptake of novel medicines. These innovative therapies are redefining standards of care. Furthermore, the increased uptake of these transformative drugs is a testament to the unwavering dedication of physicians, professionals, and the entire healthcare community in their tireless pursuit of advancing care. This momentous shift in treatment paradigms is a testament to the power of research, collaboration, and human resilience.
Mitochondrial Myopathies Pipeline Development Activities
The Mitochondrial Myopathies Therapeutics Market Report provides insights into different therapeutic candidates in Phase III, Phase II, and Phase I. It also analyzes key Mitochondrial Myopathies Companiesinvolved in developing targeted therapeutics. The report covers information on collaborations, acquisitions and mergers, licensing, and patent details for Mitochondrial Myopathies' emerging therapies.
Latest KOL Views on Mitochondrial Myopathies
To keep up with current market trends, we take KOLs and SME's opinions working in the domain through primary research to fill the data gaps and validate our secondary research. Industry Experts were contacted for insights on the mitochondrial myopathies evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake, along with challenges related to accessibility, including MD, PhD, Instructor, Postdoctoral Researcher, Professor, Researcher, and Others.
DelveInsight’s analysts connected with 10+ KOLs to gather insights; however, interviews were conducted with 6+ KOLs in the 7MM. Centers such as the California Northstate University, University of Occupational and Environmental Health, and Newcastle University, etc. were contacted. Their opinion helps understand and validate current and emerging therapy treatment patterns or mitochondrial myopathies market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
Region |
KOL Views |
|
United States |
“I have seen numerous patients with childhood onset of MELAS syndrome. Early in my career I recall two children with a disease onset before the age of 3 months with a fatal outcome. I cannot describe the lasting impact on these patients and their families and all others suffering from mitochondrial disease, especially taking into account that maternal inheritance means that all children will be a carrier of the disease.” |
|
Japan |
“We found differences in the prevalence of mitochondrial diseases among prefectures, despite the fact that the genetic background of the Japanese population is thought to be relatively uniform across the country. In addition to genetic factors, the variability in prevalence may be related to differences in health care infrastructure among prefectures in Japan. That is, there may be a concentration of patients in specific medical facilities that are well equipped for diagnosis and treatment.” |
Mitochondrial Myopathies Report Qualitative Analysis
We perform qualitative and market Intelligence analysis using various approaches, such as SWOT analysis and conjoint analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving Mitochondrial Myopathies Treatment Market Landscape.
Conjoint Analysis analyzes emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy. The analyst analyzes multiple emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. In efficacy, the trial’s primary and secondary outcome measures are evaluated.
Further, the therapies’ safety is evaluated, wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the route of administration, order of entry and designation, probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Scope of the Mitochondrial Myopathies Market Report
- The Mitochondrial Myopathies Therapeutics Market Report covers a segment of key events, an executive summary, a descriptive overview of mitochondrial myopathies, explaining its causes, signs and symptoms, pathogenesis, and currently available treatments.
- Comprehensive insight has been provided into the epidemiology segments and forecasts, the future growth potential of the diagnosis rate, and disease progression along treatment guidelines.
- Additionally, an all-inclusive account of both the current and emerging treatments, along with the elaborate profiles of late-stage and prominent therapies, will have an impact on the current treatment landscape.
- A detailed review of the Mitochondrial Myopathies Treatment Market, historical and forecasted Mitochondrial Myopathies Market Size, Mitochondrial Myopathies Drugs Market Share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The Mitochondrial Myopathies Therapeutics Market Report provides an edge while developing business strategies by understanding trends through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help in shaping and driving the 7MM Mitochondrial Myopathies Drugs Market.
Mitochondrial Myopathies Market Report Insights
- Patient-based Mitochondrial Myopathies Market Forecasting
- Therapeutic approaches
- Mitochondrial Myopathies Pipeline Analysis
- Mitochondrial Myopathies Market Size and Trends
- Existing and future Mitochondrial Myopathies Drugs Market opportunity
Mitochondrial Myopathies Market Report Key Strengths
- 10-year Mitochondrial Myopathies Market Forecast
- 7MM coverage
- Mitochondrial myopathies epidemiology segmentation
- Key cross competition
- Highly analyzed Mitochondrial Myopathies Drugs Market
- Mitochondrial Myopathies Drug Uptake
Mitochondrial Myopathies Market Report Assessment
- Current Mitochondrial Myopathies Treatment Practices
- Mitochondrial Myopathies Unmet Needs
- Mitochondrial Myopathies Drugs Profiles
- Market attractiveness
- Qualitative analysis (SWOT and conjoint analysis)
Key Questions Answered in the Mitochondrial Myopathies Market Report
Mitochondrial Myopathies Market Insights
- What was the Mitochondrial Myopathies Market Size, the market size by therapies, Mitochondrial Myopathies Drugs Market Share (%) distribution in 2024, and what would it look like by 2034? What are the contributing factors for this growth?
- What are the pricing variations among different geographies for the emerging therapies in the future?
- What can be the future treatment paradigm of mitochondrial myopathies?
- What are the disease risks, burdens, and Mitochondrial Myopathies Unmet Needs? What will be the growth opportunities across the 7MM concerning the patient population with mitochondrial myopathies?
- Who is the major future competitor in the market, and how will the competitors affect their market share?
- What are the current options for the Mitochondrial Myopathies? What are the current guidelines for treating mitochondrial myopathies in the US, Europe, and Japan?
Reasons to Buy the Mitochondrial Myopathies Market Report
- The Mitochondrial Myopathies Therapeutics Market Report will help in developing business strategies by understanding the latest trends and changing treatment dynamics driving the Mitochondrial Myopathies Drugs Market.
- Insights on patient burden/disease, Mitochondrial Myopathies Incidence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years.
- Understand the existing Mitochondrial Myopathies Drugs Market opportunities in varying geographies and the growth potential over the coming years.
- Identifying strong upcoming players in the Mitochondrial Myopathies Drugs Market will help devise strategies to help get ahead of competitors.
- Detailed analysis and ranking of class-wise potential current and emerging therapies under the conjoint analysis section to provide visibility around leading classes.
- To understand KOLs’ perspectives on the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights into the unmet needs of the existing Mitochondrial Myopathies Drugs Market so that the upcoming players can strengthen their development and launch strategy.
Stay updated with us for Recent Articles

-pipeline.png&w=256&q=75)



