Molecular Diagnostics Market Insights
Molecular Diagnostic Market, segmented by Product Type (Instruments, Reagents, and Others), Technology (Polymerase Chain Reaction [PCR], Next Generation Sequencing, In-Situ Hybridization, Microarray Analysis, and Others), Application (Infectious Disease Diagnosis, Oncology Testing, Genetic Testing, and Others), End-User (Hospitals, Pathology Labs, and Others), and Geography (North America, Europe, Asia-Pacific, and Rest of the World) is expected to advance at a substantial CAGR forecast till 2032 owing to the increasing cases of infectious diseases and the rising product developmental activities worldwide.
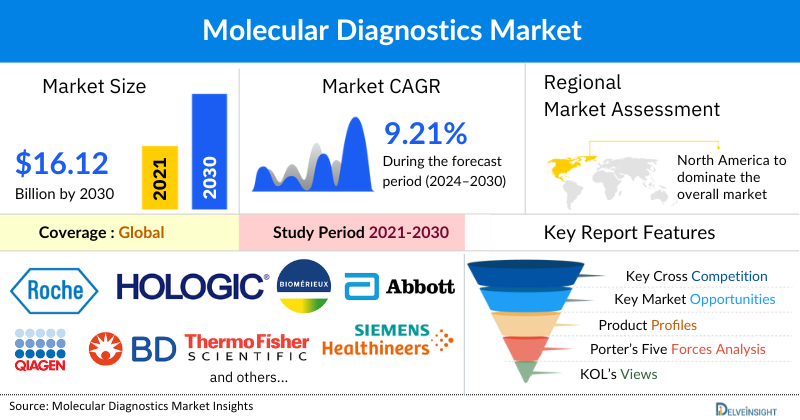
The Molecular Diagnostics Market was valued at USD 9.56 billion in 2024, growing at a CAGR of 9.21% during the forecast period from 2025 to 2032, to reach USD 16.12 billion by 2032. The molecular diagnostics market is experiencing significant growth due to the increasing cases of infectious diseases including HIV, Hepatitis B and Hepatitis C, Influenza, and human papillomavirus (HPV), introduction of new and innovative products by key Molecular Diagnostics Companies, among others, is expected to drive the Molecular Diagnostics Market from 2025 to 2032.
Molecular Diagnostic Market Dynamics
As per the World Health Organization (WHO) 2024, nearly 39 million people were living with HIV infection in 2022, globally. The source also stated that nearly 37.5 million were adults (aged 15 and above) and approximately 1.5 million people were children, aged less than 15 years. Molecular diagnostics is essential in HIV for early detection, monitoring viral load, and identifying drug resistance mutations. These tests help assess treatment efficacy, guide therapy decisions, and prevent transmission, particularly in vulnerable populations like children and adults.
Data from the WHO 2024, stated that 304 million people were infected by hepatitis B and C across the world in the year 2022. The same source further stated that out of these, 254 million were hepatitis B cases and 50 million hepatitis C cases. Molecular diagnostics play a critical role in the detection and management of hepatitis B and C by allowing for early and accurate identification of viral infections. Techniques such as PCR are used to detect viral DNA or RNA in blood samples, enabling clinicians to confirm active infections, monitor viral load, and assess the effectiveness of treatment.
Additionally, the introduction of new and innovative products by key players are pushing forward the market share for same. For example, in November 2023, Roche announced the launch of the LightCycler PRO System. The LightCycler PRO System further complemented Roche's molecular PCR testing portfolio. Hence, the interplay of all the above-mentioned factors is expected to drive the molecular diagnostics market during the given forecast period from 2025 to 2032.
However, the availability of alternative diagnostic methods, high cost associated with molecular diagnostic procedures, among others, may restrict the molecular diagnostics market growth.
Molecular Diagnostic Market Segment Analysis
Molecular Diagnostic Market is segmented by Product Type (Instruments, Reagents and Others), Technology (Polymerase Chain Reaction [PCR], Next Generation Sequencing, In-Situ Hybridization, Microarray Analysis, and Others), Application (Infectious Disease Diagnosis, Oncology Testing, Genetic Testing, and Others), End-User (Hospitals, Pathology Labs, and Others), and Geography (North America, Europe, Asia-Pacific, and Rest of the World).
In the technology segment of the molecular diagnostics market, the PCR category is expected to have a significant revenue share in the year 2024. The rapid growth of this category can be attributed to the various features and applications of PCR in diagnostics. PCR is a cornerstone of molecular diagnostics due to its precision, sensitivity, and versatility. It enables the amplification of specific DNA or RNA sequences, allowing for the detection of minute amounts of genetic material. Key features include its rapidity and ability to produce millions of copies of target sequences in a short time, making it highly useful in diagnosing infectious diseases, genetic disorders, and cancer.
PCR's accuracy stems from its specificity, as primers are designed to bind only to particular sequences, reducing false positives. Its sensitivity allows detection even at very low levels of pathogens or mutations. Real-time PCR (qPCR) extends these benefits by providing quantitative data, enabling not just the detection but also the measurement of viral or bacterial load. PCR-based diagnostics are widely used in virology, including for diseases such as COVID-19, HIV, and hepatitis. Additionally, PCR plays a crucial role in oncology for identifying gene mutations and translocations, and in genetic testing for inherited diseases. It is also applied in prenatal testing, food safety, and forensic analysis.
Therefore, owing to the above-mentioned factors, the demand for the PCR category is expected to surge, thereby the category is expected to witness considerable growth eventually contributing to the overall growth of the molecular diagnostics market during the forecast period from 2025 to 2032.
|
Report Metrics |
Details |
|
Study Period |
2022 to 2032 |
|
Base Year |
2024 |
|
Forecast Period |
2025 to 2032 |
|
Molecular Diagnostic CAGR | |
|
Molecular Diagnostic Market Size |
USD 28.44 billion by 2032 |
|
Molecular Diagnostic Companies |
|
North America is expected to dominate the Overall Molecular Diagnostic Market
Among all the regions, North America is estimated to hold a significant revenue share in the molecular diagnostics market. This domination is due to the growing burden of cancer cases in the region, rise in infectious disease instances, including Tuberculosis, growing product development activities, presence of key manufacturers, among others that are expected to fuel the molecular diagnostic market in the region during forecast period from 2024 to 2032. Global Cancer Observatory (2024), estimated that 3.83 million new case of cancer will be diagnosed by 2045 in North American region. Molecular diagnostics are used to detect genetic mutations, guide personalized treatments, and monitor disease progression through methods like liquid biopsy and companion diagnostics.
Public Health Agency of Canada (2024), stated that in 2022, there were 1,971 active tuberculosis cases in Canada. In tuberculosis, molecular diagnostics enable rapid and accurate identification of mycobacterium tuberculosis, as well as the detection of drug resistance, improving timely diagnosis and treatment. Molecular diagnostics enhance precision in both disease detection and treatment selection, ultimately improving patient outcomes. Additionally, the presence of key molecular diagnostic companies in the region and their strategic business activities will also contribute to the molecular diagnostic market growth. Rising product developmental activities by regulatory bodies in the region are likely to push forward the revenue shares for the same.
For example, in November 2023, Abbott announced that it received the U.S. Food and Drug Administration (FDA) approval for its molecular human papillomavirus or HPV screening solution. Therefore, the interplay of all the factors mentioned above would provide a conducive growth environment for the North America region in the molecular diagnostics market during the forecast period from 2024 to 2032.
Molecular Diagnostic Companies
The leading Molecular Diagnostics Companies such as Hoffmann-La Roche Ltd., Hologic, Inc., bioMérieux SA, Abbott, QIAGEN, BD, Thermo Fisher Scientific Inc., Siemens Healthineers AG, Danaher, Myriad Genetics, Inc., Agilent Technologies Inc., Illumina Inc., DiaSorin Inc., MDxHealth, Genetic Signatures, Biocartis, Exact Sciences Corporation, Amoy Diagnostics Co. Ltd., Molbio Diagnostics Pvt. Ltd., Savyon Diagnostics, and others.
Recent Developmental Activities in the Molecular Diagnostic Market:
- In April 2024, Speedx Pty Ltd. launched the new rapid polymerase chain reaction (qPCR) test for 14 different respiratory viruses in a single test that worked on almost every commercial PCR platform in less time and at a reduced cost.
- In November 2021, Roche launched the cobas® 5800 System, a new molecular laboratory instrument, in countries accepting the CE mark.
- In October 2021, Hologic launched its molecular diagnostic system, Novodiag, for on-demand molecular testing in Europe. The system combined real-time polymerase chain reaction (PCR) with microarray capabilities to enable the identification of multiple pathogens in a single sample.
- In March 2021, Materials and Machines Corporation (MatMaCorp) introduced its new handheld device MY Real-Time Analyzer (MYRTA), which could carry out polymerase chain reaction (PCR) amplification and real-time fluorescent detection.
Key Takeaways from the Molecular Diagnostic Market Report Study
- Molecular Diagnostic Market Size analysis for current market size, and market forecast for 8 years (2025-2032)
- The effect of the COVID-19 pandemic on the Molecular Diagnostic Market is significant. To capture and analyze suitable indicators, our experts are closely watching the Molecular Diagnostic market.
- Top key product/services/technology developments, merger, acquisition, partnership, joint venture happened for last 3 years
- Key Molecular Diagnostic Companies dominating the Global Market.
- Various opportunities available for the other competitor in the Molecular Diagnostic Market space.
- What are the top-performing segments in 2024? How will these segments perform in 2032.
- Which are the top-performing regions and countries in the current Molecular Diagnostic Market scenario?
- Which are the regions and countries where companies should have concentrated on opportunities for the Molecular Diagnostic market growth in the future?
Target Audience that can benefit from the Molecular Diagnostic Market Report Study
- Molecular Diagnostic providers
- Research organizations and consulting companies
- Molecular Diagnostic-related organization, association, forum, and other alliances
- Government and corporate offices
- Start-up companies, venture capitalists, and private equity firms
- Distributors and Traders in Molecular Diagnostics
- Various End-users who want to know more about the Molecular Diagnostic Market and the latest technological developments in the Molecular Diagnostic market.
Stay updated with Recent Developments @ Latest DelveInsight Blogs