Prader-Willi Syndrome Market
- Prader-Willi Syndrome Treatment Market Size in the 7MM was more than ~USD 600 million in 2023.
- Prader-Willi Syndrome is a multifaceted genetic disorder characterized by a range of challenging symptoms, including hyperphagia (excessive hunger), obesity, and behavioral issues. Prader-Willi Syndrome is often misdiagnosed in neonates because of its nonspecific clinical manifestations.
- Definitive molecular genetic testing should be readily accessible for diagnosing Prader-Willi Syndrome in the laboratory. Early Prader-Willi Syndrome diagnosis enables treatments that can greatly alleviate some of its symptoms.
- Prader-Willi Syndrome presents a significant opportunity for breakthrough treatments. An effective, approved therapy could not only extend survival but also increase the Prader-Willi Syndrome prevalence of the population over time.
- Prader-Willi Syndrome Patients are primarily managed through weight control with dietary interventions and strict supervision. Pharmacological treatment mainly involves growth hormone supplementation, which is the leading therapy due to the common growth hormone deficiency in Prader-Willi Syndrome patients.
- Growth hormone replacement therapy is the only FDA-approved treatment for children with Prader-Willi Syndrome, but it is approved solely to address growth failure and does not impact hyperphagia or behavioral issues.
- The FDA has approved three growth hormone products for Prader-Willi Syndrome: GENOTROPIN, NORDITROPIN, and OMNITROPE. In contrast, both the EMA and MHLW have only approved GENOTROPIN for Prader-Willi Syndrome. Additionally, OMNITROPE is approved as a biosimilar for Prader-Willi Syndrome in Europe.
- Prader-Willi Syndrome Companies are consistently entering the Prader-Willi Syndrome Therapeutics Market. Key Prader-Willi Syndrome Companies include Harmony Biosciences, Soleno Therapeutics, ACADIA Pharmaceuticals, Aardvark Therapeutics, Gedeon Richter, Palobiofarma, Tonix Pharmaceuticals, ConSynance Therapeutics, among others, who are working on developing therapies for Prader-Willi Syndrome.
- DCCR, developed by Soleno Therapeutics, is designed for once-daily dosing and targets hyperphagia in Prader-Willi Syndrome. As no drug is currently approved to treat hyperphagia in children and adolescents with Prader-Willi Syndrome, DCCR may capture a significant market share if it receives approval.
- Prader-Willi Syndrome Management is evolving with ongoing research and emerging treatments. Addressing current treatment gaps and advancing research is critical for improving the quality of life for individuals with Prader-Willi Syndrome.
Request for Unlocking the Sample Page of the "Prader-Willi Syndrome Treatment Market'
Key Factors Driving Prader-Willi Syndrome Market
Growing Prader–Willi Syndrome Patient Pool
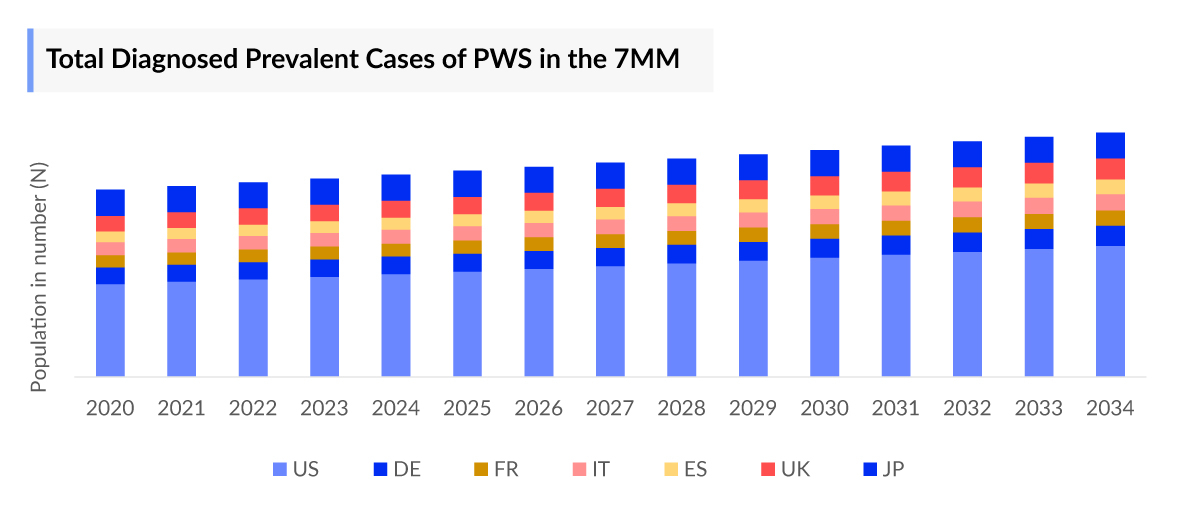
According to DelveInsight’s 2024 assessment, approximately 25,000 diagnosed prevalent cases of PWS were reported across the 7MM. This number is expected to rise through 2034, driven by improvements in diagnostic capabilities, growing awareness, and the establishment of more PWS registries that help capture undiagnosed cases.
Expanding Treatment Options with Approved Growth Hormone Therapies
The treatment landscape for PWS has strengthened with multiple FDA-approved growth hormone therapies, including GENOTROPIN, NORDITROPIN, and OMNITROPE. These therapies remain the cornerstone of care, with global approvals such as GENOTROPIN in the U.S., EU, and Japan, and OMNITROPE being available as a biosimilar in Europe, broadening patient access.
Rising Clinical Research Activity and Emerging Therapies
Several key players are advancing innovative therapies targeting unmet needs in PWS. Companies like Harmony Biosciences (WAKIX, HBS-102), ACADIA Pharmaceuticals (Carbetocin), Aardvark Therapeutics (ARD-101), Palobiofarma (PBF-999), and ConSynance Therapeutics (CSTI-500) are actively engaged in clinical development, driving momentum in the PWS pipeline.
Improved Diagnosis and Early Detection Initiatives
Increasing efforts toward newborn screening, physician education, and disease awareness campaigns are expected to enhance early recognition of PWS. By minimizing missed diagnoses at birth and enabling timely initiation of treatment, these initiatives will significantly improve patient outcomes and expand the treated patient pool over the forecast period.
DelveInsight’s "Prader–Willi Syndrome Therapeutics Market Insights, Epidemiology, and Market Forecast – 2034" report delivers an in-depth understanding of Prader-Willi Syndrome, historical and forecasted epidemiology as well as the Prader-Willi Syndrome market trends in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
The Prader-Willi Syndrome Therapeutics Market Report provides current treatment practices, marketed and emerging drugs, Prader-Willi Syndrome market share of individual therapies, and current and forecasted Prader-Willi Syndrome market size from 2020 to 2034, segmented by seven major markets. The report also covers current Prader-Willi Syndrome treatment market practices/algorithms and unmet medical needs to curate the best of the opportunities and assess the underlying potential of the market.
|
Study Period |
2020 to 2034 |
|
Forecast Period |
2024-2034 |
|
Geographies Covered |
|
|
Prader-Willi Syndrome Therapeutics Market |
|
|
Prader-Willi Syndromes Market Size | |
|
Prader-Willi Syndrome Companies |
Pfizer, Soleno Therapeutics, Neuren Pharmaceuticals, Harmony Biosciences, Acadia Pharmaceuticals, Aardvark Therapeutics, Gedeon Richter, Palobiofarma, Saniona, and others. |
|
Prader-Willi Syndrome Epidemiology Segmentation |
|
Prader–Willi Syndrome Treatment Market
Prader-Willi Syndrome is a rare genetic disorder affecting both sexes from birth throughout their lives. It results in low muscle tone, motor developmental delays, and mild to moderate learning difficulties, incomplete sexual development, and emotional and social immaturity, often leading to challenging behaviors. A chronic, insatiable appetite typically develops in childhood, leading to food seeking, stealing, and potentially life-threatening obesity without strict food management and exercise.
First described in a 1956 article by Andrea Prader, Heinrich Willi, and Alexis Labhart, Prader-Willi Syndrome was initially noted for obesity, short stature, cryptorchidism, intellectual disability, and early failure to thrive in infants. Now, Prader-Willi Syndrome is recognized as a severe neurodevelopmental disorder characterized by cognitive disabilities, behavioral issues, hyperphagia, and endocrine dysfunctions.
Prader–Willi Syndrome Diagnosis
The journey to diagnosing Prader-Willi Syndrome typically begins with parents noticing early signs such as poor muscle tone and feeding difficulties in infancy. Concerned parents consult a pediatrician, who, upon observing developmental delays and distinctive facial features, refers them to a genetic specialist. The specialist conducts a detailed clinical examination and orders genetic tests, primarily DNA methylation analysis, to confirm Prader-Willi Syndrome. Additional tests like FISH and MS-MLPA may be conducted if necessary. Once diagnosed, the specialist explains the condition, and a multidisciplinary care plan is developed, involving endocrinologists, dietitians, and therapists. Regular follow-ups and interventions are essential to manage symptoms and support the child’s development.
Further details related to diagnosis will be provided in the report…
Prader–Willi Syndrome Treatment
The Prader-Willi Syndrome treatment focuses on addressing the specific symptoms in each individual. Early intervention and strict adherence to treatment significantly enhance health and quality of life for affected individuals and their families. A coordinated effort by a team of specialists, including geneticists, pediatricians, orthopedists, endocrinologists, speech therapists, psychologists, dietitians, and nutritionists, is essential to develop an effective treatment program. Genetic counseling is recommended to discuss the condition and recurrence risks. Parents are advised to learn proper techniques for managing behavioral and eating issues, which improves prognosis. Growth hormone therapy, approved by the FDA in 2000, is beneficial for increasing height, improving body composition, mobility, and respiratory function, and may enhance development and behavior. Early intervention is crucial for addressing motor skills, intellectual disability, and speech and language development.
Prader–Willi Syndrome Epidemiology
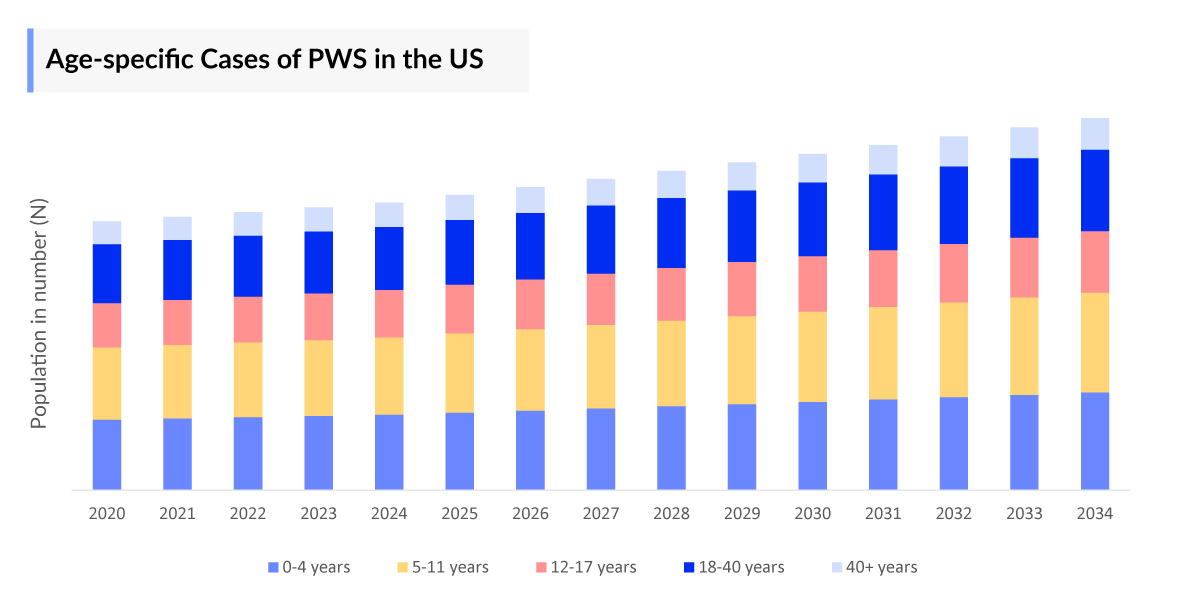
The Prader-willi syndrome epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by the total prevalent cases of Prader-Willi Syndrome, total diagnosed prevalent cases of Prader-Willi Syndrome, age-specific cases of Prader-Willi Syndrome, and Prader-Willi Syndrome cases by Genetic Subtype in the 7MM market covering the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan from 2020 to 2034.
- Among the 7MM, the US accounted for the highest Prader-Willi Syndrome Prevalence Cases in 2023, with around 22,600 cases; these cases are expected to increase during the forecast period.
- Amongst EU4 and the UK, the total Prader-Willi Syndrome Prevalence Cases were highest in Germany, while the lowest number of cases were in Spain in 2023.
- According to the estimates, in Japan, it is observed that Prader-Willi Syndrome was most prevalent in the 18-40 years age group, accounting for over 38% of total cases in 2023.
- In 2023, among genetic subtype-specific cases of Prader-Willi Syndrome in the US, the paternal microdeletion subtype accounted for the highest proportion, approximately 70% of cases, while the translocation subtype was the least common.
Prader–Willi Syndrome Market Recent Developments and Breakthroughs
- In April 2025, LogiCare3PL supported the commercial launch of VYKAT™ XR, Soleno Therapeutics' FDA-approved therapy for hyperphagia in Prader-Willi syndrome (PWS) patients. This milestone represents a significant advancement for the PWS community, with LogiCare3PL playing a key role in ensuring the therapy's accessibility.
- In April 2025, the FDA approved VYKAT™ XR, marking the first authorized treatment for hyperphagia in Prader-Willi syndrome (PWS). This milestone comes nearly 25 years after the approval of Growth Hormone Treatment (GHT) for PWS and follows rigorous FDA review backed by data from clinical trials, including a randomized withdrawal study.
- In March 2025, Soleno Therapeutics announced that the FDA approved VYKAT XR (diazoxide choline) extended-release tablets, formerly DCCR, for treating hyperphagia in adults and children 4 years and older with Prader-Willi syndrome (PWS).
- In November 2024, Soleno Therapeutics announced that the FDA has extended the review period for its New Drug Application (NDA) for DCCR (diazoxide choline) extended-release tablets, intended to treat hyperphagia in Prader-Willi syndrome (PWS) patients aged four and older.
- In Aug 2024, Soleno Therapeutics, Inc., a clinical-stage biopharmaceutical company developing novel therapeutics for rare diseases, announced that the U.S. Food and Drug Administration (FDA) has accepted its New Drug Application (NDA) for DCCR to treat Prader-Willi syndrome (Prader-Willi Syndrome) in individuals four years and older with hyperphagia.
- In July 2024, the FDA granted ConSynance Therapeutics a rare pediatric disease designation for its oral therapy aimed at treating Prader-Willi syndrome.
- In April 2024, Soleno Therapeutics, Inc. announced that the FDA granted Breakthrough Therapy Designation to diazoxide choline for the treatment of adults and children ages four years and older with genetically confirmed Prader-Willi syndrome (Prader-Willi Syndrome) who have hyperphagia.
Prader–Willi Syndrome Drugs Market Chapters
The drug chapter segment of the Prader-Willi Syndrome therapeutics market report encloses a detailed analysis of the late-stage (Phase III), mid-stage (II), and early-stage (Phase I) Prader-Willi Syndrome pipeline drugs analysis. Hormonal therapies such as GENOTROPIN, OMNITROPE, and NORDITROPIN are currently being used for treating Prader-Willi Syndrome. The current key Prader-Willi Syndrome Companies for emerging drugs and their respective drug candidates include Harmony Biosciences (pitolisant) and Soleno Therapeutics (diazoxide choline controlled release). The drug chapter also helps understand the Prader-Willi Syndrome clinical trials details, expressive pharmacological action, agreements and collaborations, approval, and patent details, and the latest news and press releases.
Prader–Willi Syndrome Marketed Drugs
-
NORDITROPIN: Novo Nordisk
NORDITROPIN (somatropin) is a recombinant human growth hormone and is developed by Novo Nordisk. NORDITROPIN is a polypeptide hormone of recombinant DNA origin. The hormone is synthesized by a special strain of E. coli bacteria that has been modified by the addition of a plasmid that carries the gene for human growth hormone. NORDITROPIN contains the identical sequence of 191 amino acids constituting the naturally occurring pituitary human growth hormone with a molecular weight of about 22,000 Daltons. In February 2018, the US FDA approved NORDITROPIN for the treatment of growth failure due to Prader-Willi Syndrome.
In February 2018, US FDA approved NORDITROPIN for the treatment of growth failure due Prader-Willi Syndrome.
Prader–Willi Syndrome Emerging Drugs
-
WAKIX (pitolisant): Harmony Biosciences
Pitolisant is a selective histamine 3 (H3) receptor antagonist/inverse agonist. The Prader-Willi Syndrome mechanism of action of WAKIX is unclear; however, its efficacy could be mediated through its activity at H3 receptors, thereby increasing the synthesis and release of histamine, a wake-promoting neurotransmitter. WAKIX was designed and developed by Bioprojet (France). Harmony has an exclusive license from Bioprojet to develop, manufacture, and commercialize pitolisant in the United States. WAKIX is FDA-approved to treat EDS or cataplexy in adult patients with narcolepsy. Pitolisant is not approved for use in Prader-Willi Syndrome patients and is currently being evaluated in Prader-Willi Syndrome. The US FDA granted Orphan Drug designation to pitolisant for the Prader-Willi Syndrome treatment in February 2024. Harmony Biosciences is also developing HBS-102 for Prader-Willi Syndrome.
In April 2024, Harmony Biosciences initiated its global Phase III registrational trial, the TEMPO study, to evaluate the safety and efficacy of pitolisant as a treatment for excessive daytime sleepiness (EDS) and behavioral symptoms in patients aged 6 years and older with Prader-Willi Syndrome.
-
Diazoxide Choline Controlled-Release (DCCR): Soleno Therapeutics
DCCR is a novel, proprietary extended-release dosage form containing diazoxide choline, the crystalline salt of diazoxide, and is administered once daily. The parent molecule, diazoxide, has been used for decades in thousands of individuals in a few rare diseases in neonates, infants, children, and adults but is not approved for use in Prader-Willi Syndrome. Soleno conceived of and established extensive patent protection for the therapeutic use of diazoxide, diazoxide choline, and DCCR in individuals with Prader-Willi Syndrome. The US FDA has granted breakthrough therapy designation to diazoxide choline for the treatment of adults and children ages 4 years and older with genetically confirmed Prader-Willi Syndrome who have hyperphagia in April 2024.
In June 2024, Soleno Therapeutics submitted the NDA to the US FDA for approval of DCCR tablets for the treatment of Prader-Willi Syndrome in individuals 4 years and older who have hyperphagia.
Prader–Willi Syndrome Drugs Market Insights
- H3-receptor antagonist/inverse agonist
Since the discovery of H3R, numerous studies have examined the clinical impact of its modulation through various drugs. Harmony Biosciences’ pitolisant, a first-in-class H3-receptor antagonist/inverse agonist, is already approved for narcolepsy and is now being evaluated for Prader-Willi Syndrome. The Phase III TEMPO study, which began in April 2024, is investigating pitolisant’s efficacy and safety for treating EDS and behavioral issues in Prader-Willi Syndrome patients aged 6 and older with Prader-Willi Syndrome.
In February 2024, the FDA granted ODD to pitolisant for Prader-Willi Syndrome. Phase II data revealed that pitolisant led to a clinically significant reduction of 3.7–5.5 points in EDS across all age groups, with the most notable effects in children aged 6–11. The drug also showed some improvement in hyperphagia, although baseline scores were generally normal to mild.
Other classes being explored in the Prader-Willi Syndrome therapeutics market include KATP channel activators, PDE-10 inhibitors, MCHR1 protein inhibitors, and others. It is too early to predict which drug will dominate the Prader-Willi Syndrome therapeutics market and demonstrate significant efficacy and safety, as many of these are new, and no results have been published yet.
Prader–Willi Syndrome Market Outlook
Current treatment options for Prader-Willi Syndrome are limited, with management primarily focusing on lifestyle modifications to prevent obesity-related deaths. Nearly half of the deaths in Prader-Willi Syndrome patients under 18 are linked to food-seeking behaviors such as choking and accidents. The clinical management of Prader-Willi Syndrome spans many therapeutic domains, including nutritional, developmental, educational, hormonal, and behavioral support, with each stage of development requiring unique management strategies. Growth hormone treatment has been shown to increase growth velocity and height, improve body composition, and, with proper dietary management, prevent obesity.
It also enhances physical and respiratory performance, thereby improving quality of life and potentially preventing long-term cardiovascular and metabolic issues such as hypercholesterolemia and diabetes. There is no cure for Prader-Willi Syndrome, but professional healthcare from a range of specialists can improve the child’s quality of life, and it cannot be prevented. Prader-Willi Syndrome treatment aims to ease some of the associated problems. First-line treatments for hyperphagia and obesity management in Prader-Willi Syndrome involve intensive behavioral and nutritional interventions.
The FDA has approved three growth hormone products for Prader-Willi Syndrome: Pfizer’s GENOTROPIN, Novo Nordisk’s NORDITROPIN, and Sandoz’s OMNITROPE. These approvals have allowed doctors to prescribe these products as equivalent treatments. The choice of growth hormone product often depends on the doctor’s experience, insurance requirements, cost, ease of use, and the patient’s history or sensitivities.
The Prader-Willi Syndrome Drugs Market composition in Europe mirrors that of the US, with growth hormone and its synthetic substitutes being the most widely used, especially among children and adolescents. EMA approved OMNITROPE as its first biosimilar growth hormone in 2006, with GENOTROPIN being another major approved growth hormone product. In March 2012, the EMA Committee for Medicinal Products for Human Use (CHMP) completed an arbitration procedure. It denied a request to add a new indication for NORDITROPIN for use in Prader-Willi Syndrome children.
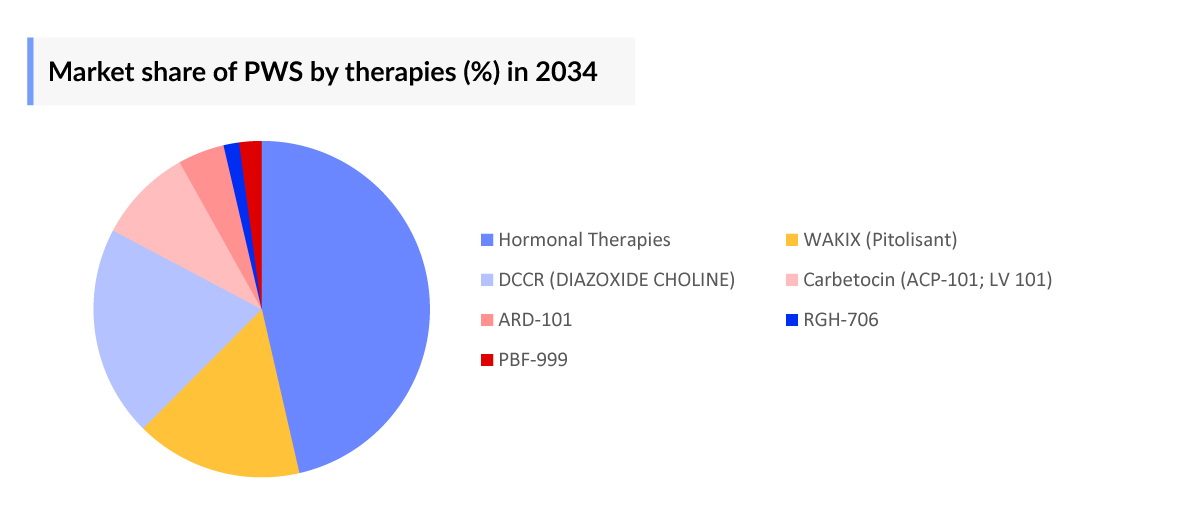
The Pipeline of Prader-Willi Syndrome Treatment includes promising drugs: DCCR (diazoxide choline), carbetocin (LV-101), pitolisant, ARD-101, and RGH-706, among others.
Key Findings
- The total Prader-Willi Syndrome Therapeutics Market Size in the US was estimated to be ~USD 500 million in 2023, which is expected to grow during the forecast period (2024–2034).
- In EU4 and the UK, Germany accounted for the largest Prader-Willi Syndrome drugs market share with ~USD 30 million market size in 2023, which is expected to grow during the forecast period (2024–2034).
- In 2034, among the emerging therapies, the highest revenue was generated by DCCR, in Japan.
- There is high uncertainty around both Gedeon Richter and Palobiofarma's therapies due to the unavailability of substantial evidence around safety & efficacy data. Meanwhile, Palobiofarma's PBF-999 first launch is expected in Spain.
Prader–Willi Syndrome Drugs Uptake
This section focuses on the uptake rate of potential Prader–Willi Syndrome drugs expected to be launched in the market during 2024–2034. The Prader-Willi Syndrome treatment market landscape has experienced a transformation with the uptake of novel drugs. These innovative therapies are redefining standards of care as growth hormones are only approved for pediatric patients. There is a high unmet need for adult patients in Prader-Willi Syndrome. More than 90% of patients have hyperphagia symptoms in Prader-Willi Syndrome, most of the companies targeting this segment.
According to analysis, significant potential Prader-Willi Syndrome drugs expected to make a substantial impact in the upcoming forecast period include DCCR (diazoxide choline), carbetocin (LV 101), and ARD-101, among others. DCCR showed promising results in these patients with first mover advantage in 2025 in the US with the highest commercial opportunity owing to its established efficacy in pivotal trials.
Prader–Willi Syndrome Pipeline Development Activities
The report provides insights into Prader–Willi Syndrome clinical trials within Phase III Phase II, and Phase I. It also analyzes key Prader-Willi Syndrome Companies involved in developing targeted therapeutics. Prader-Willi Syndrome Companies like Harmony Biosciences, Soleno Therapeutics, and ACADIA Pharmaceuticals actively engage in late-stage research and development efforts for Prader-Willi Syndrome.
Recently in May 2024, Soleno Therapeutics announced the data from its randomized withdrawal period of Study C602 of DCCR tablets in Prader-Willi Syndrome that featured in an oral presentation at the Annual Meeting of the Endocrine Society (ENDO 2024). The Prader-Willi Syndrome Pipeline possesses a potential drug and there is a positive outlook for the therapeutics market, with expectations of growth during the forecast period (2024–2034).
Pipeline Development Activities
The Prader-Willi Syndrome therapeutics maket report covers information on collaborations, acquisitions and mergers, licensing, and patent details for Prader-Willi Syndrome emerging therapy.
Latest KOL Views on Prader–Willi Syndrome
To keep up with current market trends, we take KOLs and SMEs’ opinions working in the domain through primary research to fill the data gaps and validate our secondary research. Industry Experts contacted for insights on the Prader-Willi Syndrome evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake, along with challenges related to accessibility, from experts including geneticists, pediatricians, orthopedists, endocrinologists, speech therapists, psychologists, dietitians, and nutritionists, and others.
DelveInsight’s analysts connected with 15+ KOLs to gather insights; however, interviews were conducted with 5+ KOLs in the 7MM. Universities such as the Insubria University, Professor of University of Glasgow, University of Washington, etc., were contacted. Their opinion helps understand and validate current and emerging therapy treatment patterns or Prader-Willi Syndrome therapeutics market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the Prader-Willi Syndrome drugs market and the Prader-Willi Syndrome unmet needs.
Prader-Willi Syndrome Therapeutics Market: Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT analysis and Conjoint Analysis. In SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of gaps in disease Prader-Willi Syndrome diagnosis, patient awareness, physician acceptability, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided.
Conjoint Analysis analyzes multiple approved and emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy. In efficacy, the trial’s primary and secondary outcome measures are evaluated; for instance, in event-free survival, one of the most important primary outcome measures is event-free survival and overall survival.
Further, the therapies’ safety is evaluated wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Prader-Willi Syndrome Therapeutics Market Access and Reimbursement
Life with a family member with Prader-Willi Syndrome can often mean additional expenses for the household, and any further financial help that can be obtained can be useful. Although a diagnosis of Prader-Willi Syndrome does not automatically entitle families to financial support, some allowances can be applied for.
Since 2000, the use of growth hormone has become the standard of care for individuals with Prader-Willi Syndrome. However, growth hormone is a very expensive medication, often costing USD 50,000–USD 60,000 a year at the highest dosage levels. Most families could not even consider growth hormone treatment without excellent insurance coverage or other outside funding. If a family’s insurance policy has an annual or a lifetime cap on benefits, the cost of one child’s growth hormone treatment over a long period could leave insufficient plan benefits for another family member who may need expensive care.
Health insurance plans are allowed to set their requirements for coverage, but usually, they will follow the US FDA approvals. It should not be necessary for a child with Prader-Willi Syndrome to be tested for growth hormone deficiency since the FDA’s decision regarding Prader-Willi Syndrome, effective June 20, 2000. In creating these specific “indications” for children with Prader-Willi Syndrome, the FDA recognized that GHD testing is not a reliable determinant of whether a child with Prader-Willi Syndrome needs growth hormone treatment. Those with Prader-Willi Syndrome only need to show signs of growth failure and have a genetic diagnosis of Prader-Willi Syndrome to qualify for growth hormone treatment under these special Orphan Drug Act approvals. A letter from a doctor to the insurance company might help.
OMNITROPE self-pay program
The OMNITROPE self-pay program is the most comprehensive among daily somatropin injections because it accounts for two formulation options to meet the patient’s needs. Eligible patients may save an average of over USD 5,000 per year vs. wholesale acquisition cost.
OMNITROPE copay savings program
Sandoz is committed to removing obstacles along the patient’s treatment journey. With the copay savings program, eligible patients may pay as little as USD 0 out-of-pocket costs. The up-front benefit provides immediate support for patients starting OMNITROPE. Patients are automatically re-enrolled at the start of each calendar year.
Prader–Willi Syndrome Clinical Trials Market Report Scope
- The Prader-Willi Syndrome clinical trials market report covers a segment of key events, an executive summary, and a descriptive overview, explaining its causes, signs, symptoms, pathogenesis, and currently used therapies.
- Comprehensive insight into the Prader-Willi Syndrome epidemiology segments and forecasts, disease progression, and treatment guidelines has been provided.
- Additionally, an all-inclusive account of the marketed and emerging therapies and the elaborative profiles of late-stage and prominent therapies will impact the current Prader-Willi Syndrome treatment market landscape.
- A detailed review of the Prader-Willi Syndrome clinical trials market, historical and forecasted Prader-Willi Syndrome treatment market size, Prader-Willi Syndrome drugs market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The Prader-Willi Syndrome clinical trials market report provides an edge while developing business strategies by understanding trends through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help shape and drive Prader-Willi Syndrome.
Prader–Willi Syndrome Clinical Trials Market Report Insights
- Patient-based Prader-Willi Syndrome Market Forecasting
- Prader–Willi Syndrome Therapeutic Approaches
- Prader–Willi Syndrome Pipeline Drugs Analysis
- Prader–Willi Syndrome Market Size and Trends
- Existing and Future Prader-Willi Syndrome Drugs Market Opportunity
Prader–Willi Syndrome Clinical Trials Market Report Key Strengths
- 11 Years Prader-Willi Syndrome Market Forecast
- The 7MM Coverage
- Prader-Willi Syndrome Epidemiology Segmentation
- Key Cross Competition
- Prader–Willi Syndrome Drugs Uptake
- Key Prader–Willi Syndrome Market Forecast Assumptions
Prader–Willi Syndrome Clinical Trials Market Report Assessment
- Current Prader–Willi Syndrome Treatment Market Practices
- Prader–Willi Syndrome Unmet Needs
- Prader–Willi Syndrome Pipeline Drugs Analysis Profiles
- Prader–Willi Syndrome Drugs Market Attractiveness
- Qualitative Analysis (SWOT and Analyst Views)
- Prader–Willi Syndrome Market Drivers
- Prader–Willi Syndrome Market Barriers
FAQs
- What was the Prader-Willi Syndrome clinical trials market size, the Prader-Willi Syndrome treatment market size by therapies, Prader-Willi Syndrome drugs market share (%) distribution in 2020, and what would it look like by 2034? What are the contributing factors for this growth?
- What can be the future treatment paradigm for Prader-Willi Syndrome?
- What are the disease risks, burdens, and Prader-Willi Syndrome unmet needs? What will be the growth opportunities across the 7MM concerning the patient population with Prader-Willi Syndrome?
- What are the current options for the Prader-Willi Syndrome treatment? What are the current guidelines for treating Prader-Willi Syndrome in the 7MM?
- What are the recent novel therapies, targets, mechanisms of action, and technologies being developed to overcome the limitations of existing therapies?
- What is the patient share in Prader-Willi Syndrome?
Reasons to Buy
- The Prader-Willi Syndrome clinical trials market report will help develop business strategies by understanding the latest trends and changing treatment dynamics driving Prader-Willi Syndrome.
- Insights on patient burden/disease Prader-Willi Syndrome prevalence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years.
- Understand the existing Prader-Willi Syndrome drugs market opportunities in varying geographies and the growth potential over the coming years.
- Identifying strong upcoming players in the Prader-Willi Syndrome drugs market will help devise strategies to help get ahead of competitors.
- Detailed analysis ranking of class-wise potential therapies under the analyst view section to provide visibility around leading classes.
- Highlights of access and reimbursement policies of current therapies, barriers to accessibility of expensive off-label therapies, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing Prader-Willi Syndrome drugs market so that the upcoming players can strengthen their development and launch strategy.
Stay Updated with us for Recent Articles