West Syndrome Market Summary
- The West Syndrome market in the 7MM is projected to grow at a significant CAGR by 2034 in the leading countries (US, EU4, UK and Japan).
West Syndrome Market and Epidemiology Analysis
- Among the 7MM, the maximum total diagnosed prevalent cases were found in the US in 2024.
- The majority of infants with Infantile Spasms (also known as West syndrome) present before the age of 1 year.
- West Syndrome also known as infantile spasms constitutes 1.7% of childhood epilepsies but 25% of epilepsy with onset in the first year of life. The rate of infantile spasm is estimated to be 2.5 –6.0 cases per 10,000 live births and most often occurs during the first year of life.
- According to a secondary analysis, the 10-year prevalence of west syndrome was estimated at 0.858 per 100,000 population in Japan.
- More than half of infantile spasms patients suffer from development delays and develop other seizure types as they age. While patients who achieve freedom from seizures and hypsarrhythmia can have a favorable prognosis, current medical treatment options (hormonal therapy, vigabatrin, and conventional anti-epileptic drugs) are limited and do not provide adequate relief for all Infantile Spasms patients.
- Currently, available approved treatments include SABRIL and H.P. Acthar Gel.
- Several off-label treatments are also used for symptomatic management, including nitrazepam, prednisolone, the ketogenic diet, levetiracetam, sodium valproate, topiramate, and others.
- The pipeline for west syndrome has a few products that are being developed by certain key players such as Cerecin, Amzell, Anavex Life Sciences, and others.
- Tricaprilin, an oral liquid investigational ketogenic agent, demonstrated efficacy in preclinical Infantile Spasms models. Unlike traditional ketogenic diets, it provides a potential treatment without the associated nutrient restrictions. In a Phase IIa study, Tricaprilin demonstrated a favorable safety profile, primarily causing manageable gastrointestinal side effects, and achieved ketosis in all subjects, with half experiencing reductions in spasm frequency and duration. These results suggest Tricaprilin may provide effective seizure control while reducing caregiver burden, warranting further clinical investigation.
Factors Impacting the West Syndrome Market Growth
-
Development of novel targeted therapies and biologics
Advances in gene therapy, novel antiepileptic drugs, and neuromodulation techniques are targeting the underlying causes of seizures rather than just symptoms, with emerging biologics and hormone therapies demonstrating improved efficacy in controlling seizures while minimizing side effects and reducing hospital visits
-
Integration of advanced diagnostic and monitoring technologies
Sophisticated diagnostic instruments including genetic analysis, next-generation sequencing, high-resolution imaging, and molecular diagnostics are enabling early and accurate identification of root causes, supporting personalized treatment strategies and precise disease management
-
Rising prevalence of metabolic disorders and associated risk factors
Increasing cases of metabolic disorders leading to brain energy deficiency, along with growing prevalence of risk factors such as traumatic brain injuries, genetic defects, infections, and brain malformations, are expanding the patient population requiring treatment
-
Expansion of digital health and telemedicine platforms
Wearable devices that track brain activity and seizure patterns in real-time combined with telemedicine platforms are transforming outpatient care by enabling remote consultations, continuous patient monitoring, and prompt treatment modifications, particularly improving access in underserved areas
-
High treatment costs and reimbursement challenges
Advanced diagnostics and novel therapies are associated with high costs, while limited reimbursement coverage in some regions and complexity of managing multi-disciplinary treatment approaches create barriers to market access and patient affordability
-
Increasing R&D investments and pharmaceutical collaborations
Growing investment in research and development, collaborations between pharmaceutical companies and research institutes, integration of AI-powered diagnostic tools, and approval of new treatment guidelines are accelerating innovation and expanding therapeutic options for patients
DelveInsight’s “West Syndrome – Market Insights, Epidemiology, and Market Forecast – 2034” report delivers an in-depth understanding of west syndrome, historical and forecasted epidemiology as well as west syndrome. Market trends in the United States, EU4 (Germany, France, Italy, and Spain) the United Kingdom, and Japan.
The west syndrome market report provides current treatment practices, emerging drugs, market share of individual therapies, and current and forecasted 7MM west syndrome market size from 2020 to 2034. The report also covers current west syndrome treatment practices/algorithms and unmet medical needs to curate the best opportunities and assess the market’s potential.
Scope of the West Syndrome Market Report | |
|
Study Period |
2020–2034 |
|
Forecast Period |
2025–2034 |
|
Geographies Covered |
US, EU4 (Germany, France, Italy, and Spain) the UK, and Japan |
|
West Syndrome Epidemiology |
Segmented by:
|
|
West Syndrome Key Companies |
|
|
West Syndrome Key Therapies |
|
|
West Syndrome Market |
Segmented by:
|
|
West Syndrome Analysis |
|
West Syndrome Understanding
West Syndrome Overview
West syndrome, also known as infantile spasms, is a severe epilepsy disorder that typically begins in the first year of life and is defined by a triad of symptoms: clusters of brief epileptic spasms, a distinctive chaotic EEG pattern called hypsarrhythmia, and developmental regression or delay. The spasms often involve sudden, involuntary contractions of the neck, trunk, and limbs, and may occur in clusters multiple times a day. Causes are diverse, including genetic disorders (such as tuberous sclerosis complex), brain malformations, metabolic diseases, or perinatal injury, though sometimes no cause is found. Treatment is urgent and usually involves medications like adrenocorticotropic hormone (ACTH), steroids, or vigabatrin, but despite therapy, many children experience ongoing developmental challenges and a risk of evolving into other difficult-to-treat epilepsies. Early diagnosis and intervention are crucial for improving outcomes, though the long-term prognosis often depends on the underlying cause and response to treatment.
West Syndrome Diagnosis
The diagnosis journey for West syndrome typically begins when parents or caregivers notice unusual, sudden, repetitive movements in their infant—such as brief, jerky spasms of the neck, trunk, or limbs, often occurring in clusters, especially upon waking or before sleep. Concerned, they consult a pediatrician, who may initially mistake these subtle spasms for benign conditions like colic. If West syndrome is suspected, the physician will request a detailed seizure history and encourage video documentation of the episodes to help distinguish them from other disorders. The next step is an urgent referral to a pediatric neurologist, who will perform an EEG; a hallmark of West syndrome is the presence of hypsarrhythmia—a chaotic, high-amplitude EEG pattern. Further investigations, such as brain MRI and metabolic or genetic tests, may be conducted to identify underlying causes like tuberous sclerosis or metabolic disorders. Once confirmed, prompt initiation of treatment—typically with adrenocorticotropic hormone (ACTH), steroids, or vigabatrin—is crucial to improve outcomes and reduce the risk of long-term developmental delays. Throughout this journey, early recognition and rapid intervention are key, as delays in diagnosis or treatment can lead to worse neurological outcomes for the child.
Further details related to diagnosis are provided in the report…
West Syndrome Treatment
The treatment of west syndrome is a medical emergency, with prompt intervention being critical to reduce the risk of irreversible neurodevelopmental impairment. The mainstay of therapy involves hormonal treatments and antiseizure medications, often initiated simultaneously or in sequence based on the clinical context. The two most established first-line therapies are adrenocorticotropic hormone (ACTH) and oral corticosteroids (e.g., prednisolone). Recently, synthetic peptide analogues, such as AMZ002, have been developed to replicate the therapeutic effects of ACTH with potentially improved safety and tolerability. Vigabatrin is the preferred agent in patients with tuberous sclerosis complex, where it has demonstrated superior efficacy in controlling spasms. In cases where first-line therapies are ineffective, other antiepileptic drugs such as valproic acid, topiramate, or zonisamide may be considered. The ketogenic diet, a high-fat, low-carbohydrate dietary regimen, has also shown benefit in controlling seizures in drug-resistant cases. For children with a localized epileptogenic focus identifiable on neuroimaging, surgical intervention—such as focal cortical resection or hemispherectomy—may be curative. Treatment selection should be individualized, guided by the underlying etiology, clinical severity, and response to initial therapy. Early recognition and aggressive treatment are strongly correlated with improved seizure control, neurodevelopmental outcomes, and long-term quality of life.
Further details related to treatment are provided in the report…
West Syndrome Epidemiology
The west syndrome epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by diagnosed prevalent cases of DEE, diagnosed prevalent cases of west syndrome, treatable cases of west syndrome in the 7MM covering the United States, EU4 (Germany, France, Italy, and Spain), United Kingdom, and Japan from 2020 to 2034.
Key Findings from the West Syndrome Epidemiology Analysis
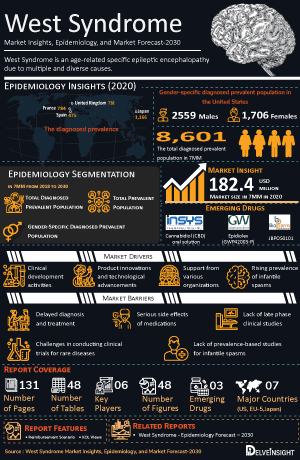
- Total diagnosed prevalent cases of DEE in the 7MM in 2024 were approximately 290,000.
- Among the 7MM, the US accounted for the highest diagnosed prevalent cases of west syndrome in 2024.
- The total diagnosed prevalent cases of west syndrome in the US in 2024 was approximately 8,000.
- Among the EU4 and UK, the maximum total diagnosed prevalent cases were found in Germany and the lowest were in Spain in 2024.
- In Japan, the total diagnosed prevalent cases were found to be approximately 3,400 in 2024.
West Syndrome Epidemiology Segmentation
- Diagnosed prevalent cases of DEE
- Diagnosed prevalent cases of west syndrome
- Treatable cases of west syndrome
West Syndrome Drug Analysis
The drug chapter segment of the west syndrome report encloses a detailed analysis of west syndrome marketed drugs and late-stage (Phase III and Phase II) west syndrome pipeline drugs. It also helps to understand the west syndrome clinical trial details, expressive pharmacological action, agreements and collaborations, approval and patent details, advantages and disadvantages of each included drug, and the latest news and press releases.
West Syndrome Marketed Drugs
H.P. ACTHAR GEL: Mallinckrodt Pharmaceuticals/Questcor Pharmaceuticals
H.P. ACTHAR GEL is an adrenocorticotropic hormone (ACTH) analog indicated as monotherapy for the treatment of infantile spasms in infants and children under 2 years of age and also indicated for the treatment of exacerbations of multiple sclerosis in adults. This gel was approved by the FDA in 1952 and has been used to treat infantile spasms for more than 50 years. It is recommended by the American Academy of Neurology and the Child Neurology Society as a treatment for infantile spasms. It was previously approved for various indications, including acute multiple sclerosis exacerbations and nephrotic syndrome. It is a purified preparation of ACTH obtained from pigs’ pituitary glands.
West Syndrome Emerging Drugs
Tricaprilin (CER-0001): Cerecin Neurosciences
Tricaprilin is an investigational oral drug that, in normal dietary conditions, is believed to mimic ketosis after administration. Tricaprilin elevates plasma ketone levels and is thereby intended to leverage the numerous activities and benefits of ketone bodies. Ketogenic diets have been used for decades to treat a variety of seizure disorders, including different pediatric epilepsies and infantile spasms, otherwise known as West syndrome. Yet, despite a great deal of work in this area, the precise mechanisms underlying the ketogenic diet’s clinical effects in such a wide array of seizure disorders remain unclear.
In October 2020, Cerecin announced that the US FDA has granted an Orphan Drug Designation to tricaprilin for the treatment of infantile spasms (also known as West’s syndrome), a rare form of childhood epilepsy. The company reported promising results from the 2023 Pilot Phase IIa study and is preparing to initiate a global Phase III pivotal trial.
ANAVEX2-73 (Blarcamesine): Anavex Life Sciences
ANAVEX2-73 developed by Anavex Life Sciences, is a novel investigational agent currently in Phase 1 clinical development for the treatment of Infantile Spasms and has received Orphan Drug Designation from the US FDA for this indication. The compound acts as a sigma-1 receptor agonist and a muscarinic receptor modulator, targeting key mechanisms implicated in the pathophysiology of early-onset epileptic encephalopathies. The drug is currently being investigated in Phase I. According to the company’s pipeline, Phase II and III trials are planned.
|
Comparison of Emerging Drugs Under Development for West Syndrome | ||||||
|
Product |
Company |
Mechanism of Action |
Phase |
Indication |
ROA |
Molecular Type |
|
Tricaprilin (CER-0001) |
Cerecin |
Induce ketogenesis |
Currently Phase II, Planned global Phase III trial |
Infantile spasms |
Oral |
Small molecule |
|
AMZ002* |
Amzell |
Hormone replacements |
III |
Infantile spasms |
Injectable solution |
Synthetic polypeptide |
|
ANAVEX2-73 (blarcamesine) |
Anavex Life Sciences |
Targeting sigma-1 and muscarinic receptors |
I |
Infantile spasms |
Oral |
Small-molecule |
|
NOTE *-As per the pipeline, the drug is in Phase III, but the Phase III trial for Infantile Spasms participants was withdrawn due to a strategic decision. | ||||||
Note: Detailed list will be provided in the final report.
West Syndrome Market Outlook
The current treatment landscape for West Syndrome primarily includes marketed therapies like Hormonal Therapy (ACTH), such as H.P. ACTHAR GEL, and SABRIL (vigabatrin), alongside off-label options like corticosteroids, which are commonly used as first-line treatments. Anti-epileptic drugs (AEDs) are typically prescribed as second-line treatments. Among the first-line therapies, ACTH is widely recognized as the most effective and universally accepted option for treating West Syndrome.
Looking forward, the market for West Syndrome is expected to expand significantly with the introduction of emerging therapies. Notably, Tricaprilin, was developed by Cerecin Neurosciences. This therapy is expected to offer new treatment options, potentially addressing the limitations of existing therapies and improving patient outcomes. As a result, the market is projected to see growth in both treatment choices and market revenue during this forecast period. Enhanced awareness, early diagnosis, and ongoing clinical advancements will likely contribute to the increasing demand for more effective therapies in the coming years. According to DelveInsight, the introduction of novel treatments is expected to reshape the west syndrome market in the 7MM during the forecast period.
- The US accounts for the largest market size of west syndrome, in comparison to EU4 and the UK (Germany, France, Italy, the UK, and Spain) and Japan in 2024.
- Among the EU4 and the UK, Germany had the highest market size, while Spain had the lowest market size for west syndrome in 2024.
- Anti-epileptic drugs (AEDs) like vigabatrin and corticosteroids are currently first-line treatments for west syndrome. However, researchers are focused on developing more targeted therapies that aim to address the underlying causes of the disorder.
- The emerging landscape for West syndrome is marked by a few treatment options in development, with the expected launch of these therapies poised to drive significant market growth in the coming years.
West Syndrome Drugs Uptake
This section focuses on the uptake rate of potential drugs expected to be launched in the market during 2025–2034. The treatment landscape for West Syndrome remains relatively underdeveloped, with limited drug options currently available in the market and few emerging therapies on the horizon. West Syndrome continues to be managed primarily through established treatment approaches with minimal changes over recent years. The absence of a robust pipeline highlights the urgent need for increased research efforts and pharmaceutical investment. Despite these limitations, healthcare professionals, including pediatric neurologists and epilepsy specialists, continue to strive for better outcomes through early diagnosis and tailored care. While transformative therapies are not yet within reach, the commitment of the medical community remains a driving force in advocating for advancements in this challenging and rare epileptic disorder.
Further detailed analysis of emerging therapies drug uptake in the report…
West Syndrome Clinical Trials Analysis
The report provides insights into therapeutic candidates in Phase III, Phase II, and Phase I. It also analyzes key players involved in developing targeted therapeutics.
Pipeline Development Activities
The report covers information on collaborations, acquisitions, mergers, licensing, and patent details for West Syndrome emerging therapy.
Latest KOL Views on West Syndrome
To keep up with current market trends, we take KOLs and SMEs’ opinions working in the domain through primary research to fill the data gaps and validate our secondary research. Industry Experts were contacted for insights on West Syndrome's evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake, along with challenges related to accessibility and others.
Delveinsight’s analysts connected with 10+ KOLs to gather insights; however, interviews were conducted with 5+ KOLs in the 7MM. Centers such as Medicine at Harvard Medical School in the US, Boston Children’s Hospital in the US, Oxford University in the UK, the University of California, San Francisco, and the University of Heidelberg in Germany. Their opinion helps understand and validate current and emerging therapy treatment patterns or West Syndrome market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
What KOLs are saying on West Syndrome Patient Trends? |
|
“West Syndrome is a devastating pediatric epilepsy with long-term developmental consequences. Standard therapies like ACTH and vigabatrin are often effective but not curative. The US market is poised for growth as novel therapies—including gene-targeted treatments and neuroprotective agents—move through the pipeline. Increased awareness and early diagnosis are expanding treatment windows and driving clinical investment.” – Boston Children’s Hospital, the US |
|
“Treatment outcomes for West Syndrome vary widely, depending on etiology and timing. Germany’s advanced diagnostics and healthcare infrastructure create ideal conditions for early detection and therapeutic innovation. Interest in next-gen therapies such as stem-cell-based interventions is growing, pointing to a dynamic and promising market.” – University of Heidelberg, Germany |
West Syndrome Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT analysis and conjoint analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the Analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving treatment landscape.
Conjoint Analysis analyzes multiple approved and emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy.
In efficacy, the trial’s primary and secondary outcome measures are evaluated; for instance, in event-free survival, one of the most important primary outcome measures is event-free survival and overall survival.
Further, the therapies’ safety is evaluated, wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the probability of success and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
West Syndrome Market Access and Reimbursement
West Syndrome patients have to face a huge economic burden alone without any healthcare coverage or proper reimbursement policies.
Reimbursement may be referred to as the negotiation of a price between a manufacturer and payer that allows the manufacturer access to the market. It is provided to reduce the high costs and make the essential drugs affordable. Health technology assessment (HTA) plays an important role in reimbursement decision-making and recommending the use of a drug. These recommendations vary widely throughout the seven major markets, even for the same drug. In the US healthcare system, both Public and Private health insurance coverage are included. Also, Medicare and Medicaid are the largest government-funded programs in the US. The major healthcare programs, including Medicare, Medicaid, Health Insurance Program (CHIP), and the state and federal health insurance marketplaces, are overseen by the Centers for Medicare & Medicaid Services (CMS). Other than these, Pharmacy Benefit Managers (PBMs), and third-party organizations that provide services and educational programs to aid patients are also present.
The report further provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of currently used therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Further detailed analysis of emerging therapies drug uptake in the report…
Scope of the West Syndrome Market Report
- The report covers a segment of key events, an executive summary, and a descriptive overview of west syndrome, explaining its causes, signs, symptoms, pathogenesis, and currently used therapies.
- Comprehensive insight into the epidemiology segments and forecasts, disease progression, and treatment guidelines has been provided.
- Additionally, an all-inclusive account of the emerging therapies and the elaborative profiles of late-stage and prominent therapies will impact the current treatment landscape.
- A detailed review of the west syndrome market, historical and forecasted market size, market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The report provides an edge while developing business strategies, by understanding trends, through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help shape and drive the 7MM west syndrome market.
West Syndrome Market Report Insights
- Patient Population
- Therapeutic Approaches
- West Syndrome Pipeline Analysis
- West Syndrome Market Size and Trends
- Existing and Future Market Opportunity
West Syndrome Market Report Key Strengths
- 9 Years Forecast
- The 7MM Coverage
- West Syndrome Epidemiology Segmentation
- Key Cross Competition
- Drugs Uptake and Key Market Forecast Assumptions
West Syndrome Market Report Assessment
- Current Treatment Practices
- Unmet Needs
- Pipeline Product Profiles
- Market Attractiveness
- Qualitative Analysis (SWOT Analysis and Conjoint Analysis)
Key Questions Answered in the West Syndrome Market Report
- What was the west syndrome market size, the market size by therapies, market share (%) distribution in 2024, and what would it look like by 2034? What are the contributing factors for this growth?
- What are the pricing variations among different geographies for approved therapies?
- What can be the future treatment paradigm of west syndrome?
- What are the disease risks, burdens, and unmet needs of west syndrome? What will be the growth opportunities across the 7MM concerning the patient population with West Syndrome?
- What are the current options for the treatment of west syndrome? What are the current guidelines for treating west syndrome in the US, Europe, and Japan?
- What are the recent novel therapies, targets, mechanisms of action, and technologies being developed to overcome the limitations of existing therapies?
Reasons to Buy West Syndrome Market Report
- The report will help develop business strategies by understanding the latest trends and changing treatment dynamics driving the West Syndrome market.
- Insights on patient burden/disease prevalence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years.
- Understand the existing market opportunities in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
- Identifying upcoming players in the market will help devise strategies to help get ahead of competitors.
- Detailed analysis and ranking of class-wise potential current and emerging therapies under the Analyst view section to provide visibility around leading classes.
- Highlights of access and reimbursement policies of current therapies, barriers to accessibility of expensive off-label therapies, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing market so that the upcoming players can strengthen their development and launch strategy.