ANCA associated Vasculitis Market
- The total ANCA associated Vasculitis market size in the 7MM was around USD 1,500 million in 2023, and the market is expected to grow significantly over the forecast period owing to launch of new therapies such as Benralizumab (AstraZeneca), depemokimab (GlaxoSmithKline), and others.
- The vasculitides are a heterogeneous group of conditions typified by their ability to cause vessel inflammation with or without necrosis.
- ANCA associated Vasculitis are a heterogeneous group of rare autoimmune conditions that causes an inflammation of blood vessels with various manifestations.
- It includes three main diseases, which are granulomatosis with polyangiitis (GPA; formerly known as Wegener granulomatosis), eosinophilic granulomatosis with polyangiitis (EGPA; previously known as Churg-Strauss syndrome), and microscopic polyangiitis (MPA).
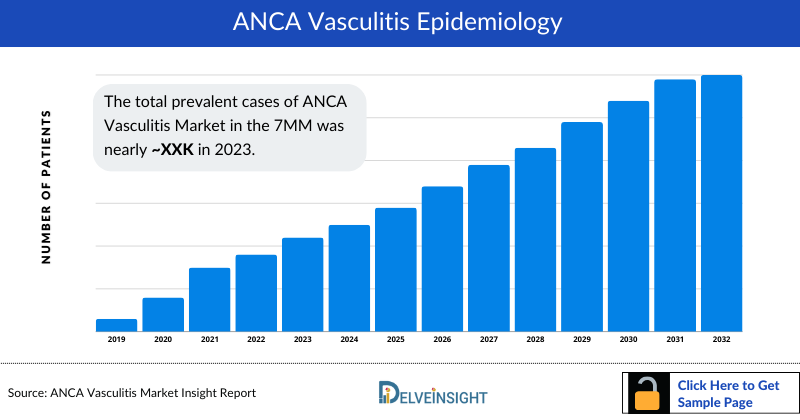
- As per DelveInsight’s estimates, the total diagnosed prevalent cases of ANCA associated Vasculitis were ~214,800 in the 7MM in 2023. Among the 7MM, the US contributed highest number of cases, followed by Japan.
- Among three types of ANCA associated Vasculitis, Granulomatosis with Polyangiitis (GPA) was more prevalent compared to Microscopic Polyangiitis (MPA) and Eosinophilic Granulomatosis with Polyangiitis (EGPA).
- Although the incidence of MPA is higher as compared to GPA, but the prevalence is much lower than GPA as patients of MPA present severe manifestations at the time of diagnosis leading to high mortality rate and lower recorded prevalence.
- Triggers for developing ANCA associated Vasculitis include microbial infections, a reaction to certain medications, certain genetic variations, or exposure to toxins.
- The treatment of ANCA associated Vasculitis consists of two phases: remission-induction and remission-maintenance. Usually, remission-induction treatment starts with cyclophosphamide, rituximab, and high-dose steroids, whereas maintenance of remission is done by either methotrexate or azathioprine.
- Current therapies are often effective in inducing and maintaining remission but are associated with a range of toxicities.
- In the patients with relapsing/refractory disease, those who do not respond to cyclophosphamide or glucocorticoids, rituximab, NUCALA (in case of GPA/MPA), and TAVNEOS (in case of EGPA) are prescribed.
- In 2021, the US FDA approved TAVNEOS (avacopan) as an adjunctive treatment of adult patients with severe active ANCA associated Vasculitis, specifically GPA and MPA in combination with standard therapy.
- Patients with ANCA associated Vasculitis encounter delays in obtaining an accurate diagnosis which can lead to substantial morbidity and increased mortality. Therefore, there is a huge need for effective and early diagnosis of ANCA associated Vasculitis and a reliable biomarker which can help in early detection of the disease.
Request for Unlocking the CAGR of the "ANCA Vasculitis Drug Market"
ANCA-associated Vasculitis Market Report Summary
- The ANCA associated Vasculitis market report offers extensive knowledge regarding the epidemiology segments (by region, total diagnosed prevalent cases of ANCA associated Vasculitis, prevalent cases of ANCA associated Vasculitis by type, prevalent cases of ANCA associated Vasculitis by organ involvement, prevalent cases of ANCA associated Vasculitis by antibody type, prevalent cases of ANCA associated Vasculitis by severity, and total treated cases) and predictions, presenting a deep understanding of the potential future growth in diagnosis rates, disease progression, and treatment guidelines. It provides comprehensive insights into these aspects, enabling a thorough assessment of the subject matter.
- Additionally, an all-inclusive account of the current management techniques and emerging therapies such as Depemokimab (GSK3511294), FASENRA (benralizumab) and the elaborative profiles of late and mid-stage (Phase III and Phase II) and prominent therapies that would impact the current treatment landscape and result in an overall market shift has been provided in the report.
- The report also encompasses a comprehensive analysis of the ANCA associated Vasculitis market, providing an in-depth examination of its historical and projected market size (2020–2034). It also includes the market share of therapies, detailed assumptions, and the underlying rationale for our methodology. The report also includes drug outreach coverage in the 7MM region.
- The report includes qualitative insights that provide an edge while developing business strategies, by understanding trends, through SWOT analysis and expert insights/KOL views, including experts from various hospitals and prominent universities, patient journey, and treatment preferences that help shape and drive the 7MM ANCA associated Vasculitis market.
The table given below further depicts the key segments provided in the report:
|
Study Period |
2020 to 2034 |
|
Forecast Period |
2024-2034 |
|
Geographies Covered |
The US, EU4 (Germany, France, Italy, and Spain) and UK, Japan |
|
ANCA Associated Vasculitis Market |
|
|
ANCA Associated Vasculitiss Market Size | |
|
ANCA Associated Vasculitis Companies |
GlaxoSmithKline, AstraZeneca, InflaRx, Staidson BioPharma, Bio-Thera Solutions, and others. |
|
ANCA Associated Vasculitis Epidemiology Segmentation |
|
ANCA-associated Vasculitis Market
Various key players such as GlaxoSmithKline, AstraZeneca, InflaRx and others, are involved in developing therapies for ANCA associated Vasculitis. Expected launch of emerging therapies and other treatments, will lead to a significant increase in the market size during the forecast period [2024–2034].
- According to DelveInsight estimates, the United States accounted for the highest market size among the 7MM in 2023 followed by Japan for ANCA associated Vasculitis.
- As per DelveInsight’s patient-based forecasting model, NUCALA (mepolizumab) and rituximab accounted for the highest share among available therapies in 2023 in the 7MM.
- In the 7MM, TAVNEOS (avacopan) is expected to generate the maximum revenue, more than USD 1 billion in 2034.
- Among emerging therapies such as FASENRA (benralizumab) and GSK3511294 (depemokimab), and others, FASENRA is expected to generate more revenue compared to other therapies.
Also Read- BENRALIZUMAB Drug Insight
ANCA associated Vasculitis Drug Chapters
The section dedicated to drugs in the ANCA associated Vasculitis market report provides an in-depth evaluation of pipeline drugs (Phase III, and Phase II) related to ANCA associated Vasculitis.
The drug chapters section provides valuable information on various aspects related to clinical trials of ANCA associated Vasculitis, such as the pharmacological mechanisms of the drugs involved, designations, approval status, patent information, and a comprehensive analysis of the pros and cons associated with each drug. Furthermore, it presents the most recent news updates and press releases on drugs targeting ANCA associated Vasculitis.
Marketed ANCA associated Vasculitis Therapies
TAVNEOS (avacopan): Chemocentryx/Amgen
TAVNEOS (avacopan), approved by the FDA as an adjunctive treatment for adults with severe active ANCA-associated vasculitis, is a first-in-class, orally administered small molecule that employs a novel, highly targeted mode of action in complement-driven autoimmune and inflammatory diseases. While the precise mechanism in ANCA-associated vasculitis has not been definitively established, TAVNEOS, by blocking the complement 5a receptor (C5aR) for the pro-inflammatory complement system fragment known as C5a on destructive inflammatory cells such as blood neutrophils, is presumed to arrest the ability of those cells to do damage in response to C5a activation, which is known to be the driver of ANCA associated Vasculitis.
In January 2022, Vifor Fresenius Medical Care Renal Pharma (VFMCRP) announced that the European Commission had approved TAVNEOS in combination with a rituximab or cyclophosphamide regimen for the treatment of adult patients with severe, active GPA or MPA. TAVNEOS received marketing authorization in all European Union member states, as well as in Iceland, Liechtenstein, and Norway
In March 2023, Vifor Fresenius Medical Care Renal Pharma (VFMCRP) announced that TAVNEOS has been included in the revised EULAR ANCA associated Vasculitis management recommendations, as one of several important updates in the 2022 version (CSL Vifor, 2023).
Note: Detailed assessment will be provided in the final report of ANCA associated Vasculitis…
Check More @ TAVNEOS Drug Insight
Emerging ANCA associated Vasculitis Therapies
Depemokimab (GSK3511294): GlaxoSmithKline
Depemokimab, known as GSK3511294, is a humanized anti-IL-5 mAb (immunoglobulin G1, kappa) that provides an extended half-life and improved affinity for IL-5 compared with approved anti-IL-5 mAbs. As it binds to the same epitope as mepolizumab, these modifications are not expected to change the clinical efficacy and safety profile but instead confer a longer duration of action. In a cell-based in vitro assay, depemokimab demonstrated an approximately 29-fold increase in IL-5 potency vs. mepolizumab. This longer duration of action may reduce the frequency of dosing required and improve patient convenience, leading to greater treatment compliance.
Currently, it is being evaluated in Phase III (NCT05263934; OCEAN) clinical trial to investigate the efficacy and safety of depemokimab compared with mepolizumab in adults with relapsing or refractory EGPA receiving SoC therapy. The data is anticipated to be released after 2025 (GSK, 2023).
FASENRA (benralizumab): AstraZeneca
FASENRA (benralizumab), developed by AstraZeneca, is a monoclonal antibody that binds directly to IL-5 receptor alpha on eosinophils and attracts natural killer cells to induce rapid and near-complete depletion of blood and tissue eosinophils in most patients via apoptosis (programmed cell death) (AstraZeneca, 2021).
The drug is being evaluated in Phase III (NCT04157348; MANDARA) clinical trial to compare the efficacy and safety of benralizumab 30 mg vs. mepolizumab 300 mg administered by SC injection in patients with relapsing or refractory EGPA on corticosteroid therapy with or without stable immunosuppressive therapy.
In September 2023, positive high-level results from the MANDARA (Phase III trial) for FASENRA demonstrated non-inferior rates of remission compared to mepolizumab in patients with EGPA who were receiving oral corticosteroids with or without stable immunosuppressive therapy. MANDARA was the first head-to-head trial of biologics in EGPA, comparing a single monthly injection of FASENRA to three injections per month of mepolizumab, the only currently approved treatment (AstraZeneca, 2023b).
Take Your Research to the Next Level! Click Here to Get Access to the Full Pipeline Report @ ANCA Vasculitis Drugs
Note: Detailed assessment will be provided in the final report of ANCA associated Vasculitis…
ANCA associated Vasculitis Market Outlook
Current treatment guidelines for ANCA associated Vasculitis emphasize stopping vasculitic activity, preventing vasculitis from returning, and addressing longer-term comorbidities caused by tissue damage, drug toxicity, and increased cardiovascular and malignancy risk. The current treatment pathway for most patients involves induction with cyclophosphamide or rituximab in combination with glucocorticoids, followed by a maintenance phase with rituximab, azathioprine, or methotrexate, during which time glucocorticoids are tapered. Current therapies are often effective in inducing and maintaining remission but are associated with various toxicities.
Given the efficacy and comparable safety profile of rituximab, it is often chosen for remission induction. The most common regimens include 375 mg/m2 weekly for 4 weeks or 1,000 mg twice over 2 weeks. Cyclophosphamide remains a cornerstone of therapy for many providers, especially where rituximab is prohibitively expensive. The relatively short follow-up in RAVE limited an assessment of differences in long-term outcomes, such as malignancy and survival, between the two treatment arms, but recent observational data suggest that rituximab, in contrast to cyclophosphamide, is not associated with an increased risk of malignancy.
Many other new molecules, a few with novel mechanisms, like belimumab (B-cell activating factor inhibitors), sparsentan (angiotensin type 1 receptor antagonists), and NS-229 (Selective JAK1 inhibitor), among others, are also being investigated in earlier stages of development for the treatment of ANCA associated Vasculitis.
In a nutshell, a few potential therapies are being investigated for the management of ANCA associated Vasculitis. Even though it is too soon to comment on the above-mentioned promising candidate to enter the market during the forecast period (2024–2034), it is safe to assume that the future of this market is bright. Eventually, the drug shall create a significant difference in the treatment landscape of ANCA associated Vasculitis in the coming years. The treatment space is expected to experience a positive impact in the coming years owing to the improvement in the rise in the number of healthcare spending across the world.
See Here @ NUCALA Drug Insight
ANCA associated Vasculitis Disease Understanding and Treatment
ANCA associated Vasculitis Overview
The vasculitides are defined by inflammatory leukocytes in vessel walls with reactive damage to mural structures. Loss of vessel integrity leads to bleeding and compromise of the lumen, which may result in downstream tissue ischemia and necrosis. In general, affected vessels vary in size, type, and location associated with the specific type of vasculitis. Vasculitis may occur as a primary process or secondary to another underlying disease.
ANCA-associated vasculitis is an umbrella term for relatively rare autoimmune diseases of unknown cause, characterized by inflammatory cell infiltration causing necrosis of blood vessels. They are often associated with the production of antibodies that target neutrophil antigens. The two major antigens targeted by ANCAs are leukocyte proteinase 3 (PR3) and myeloperoxidase (MPO). The three types of small-vessel vasculitis, namely granulomatosis with polyangiitis (GPA), microscopic polyangiitis (MPA), and eosinophilic GPA (EGPA), feature a loss of tolerance to neutrophil primary granule proteins, most often leukocyte proteinase 3 (PR3; also known as myeloblastin) or MPO.
Further details are provided in the report…
ANCA associated Vasculitis Diagnosis
No test can confirm the presence of ANCA associated Vasculitis. To help the doctor reach a diagnosis, the doctor will observe the symptoms and note down the medical history of the patient. The doctors can also conduct a physical examination, partly focusing on the arteries in the temples, and if the patient has ANCA associated Vasculitis, the arteries may feel hard or tender.
Then, the patients are required to go through some testing procedures. Typically, if a doctor suspects the ANCA associated Vasculitis, the first tests ordered are blood tests looking for inflammation. These may include Erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) blood tests; high levels of either signify inflammation suggesting ANCA associated Vasculitis. While blood tests and imaging are helpful, there is one test often prized above all others. The gold-standard test for ANCA associated Vasculitis has traditionally been a temporal artery biopsy showing active inflammation of the temporal artery. If a doctor recommends a patient that they should have a temporal artery biopsy, this generally means they have a high level of suspicion for ANCA associated Vasculitis.
Further details related to country-based variations are provided in the report…
ANCA associated Vasculitis Treatment
The treatment of ANCA associated Vasculitis consists of two phases: remission-induction and remission-maintenance. Inducing long-term remission is one of the main goals of immunosuppressive therapy, which might be disrupted by the clinical reappearance of disease activity (relapse). For this reason, after controlling disease manifestations, remission-maintenance treatment is instituted.
Treatment for induction of remission, is usually done by cyclophosphamide, rituximab, and high-dose steroids. Sometimes with a life-threatening disease or severe kidney involvement, plasma exchange is used along with induction treatment. Maintenance of remission is usually done by either methotrexate or azathioprine. Although there is no consensus on the duration of maintenance, it is usually given for 18–24 months to avoid relapse. Additionally, rituximab is recommended to maintain remission in patients with GPA and MPA.It is recommended to use glucocorticoids plus cyclophosphamide or rituximab in an organ or life-threatening disease. Methotrexate or mycophenolate mofetil are recommended for non-organ-threatening diseases.
Further details related to treatment and management are provided in the report…
Know more @ FASENRA Drug Insight
ANCA associated Vasculitis Epidemiology
The ANCA associated Vasculitis epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by diagnosed prevalent cases, type-specific cases, organ involvement-specific cases, antibody-specific cases, severity-specific cases and total treated cases in the United States, EU4 countries (Germany, France, Italy, Spain) and the United Kingdom, and Japan from 2020 to 2034.
- Among the 7MM, United States accounted for the highest number of diagnosed prevalent cases of ANCA associated Vasculitis in 2023.
- In 2023, the diagnosed prevalent cases of MPA by organ involvement in the US were highest for renal impairment, followed by lower respiratory tract, cutaneous manifestation, gastrointestinal, nervous system, upper respiratory tract, cardiovascular, and eye involvement.
- In EU4 and the UK, out of all diagnosed prevalent cases of ANCA associated Vasculitis by type, GPA accounted for highest number of cases followed by MPA and EGPA respectively in 2023.
- In 2023, out of all diagnosed prevalent cases of ANCA associated Vasculitis by type in Japan, MPA accounted for highest number of cases followed by GPA and EGPA respectively.
Further details related to epidemiology will be provided in the report…
Unlock comprehensive insights! Click Here to Purchase the Full Report @ ANCA Vasculitis Prevalence
KOL Views
To stay abreast of the latest trends in the market, we conduct primary research by seeking the opinions of Key Opinion Leaders (KOLs) and Subject Matter Experts (SMEs) who work in the relevant field. This helps us fill any gaps in data and validate our secondary research.
We have reached out to industry experts to gather insights on various aspects of ANCA associated Vasculitis, including the evolving treatment landscape, patients’ reliance on conventional therapies, their acceptance of therapy switching, drug uptake, and challenges related to accessibility. The experts we contacted included medical/scientific writers, professors, and researchers from prestigious universities in the US, Europe, the UK, and Japan.
Our team of analysts at Delveinsight connected with more than 10 KOLs across the 7MM. By obtaining the opinions of these experts, we gained a better understanding of the current and emerging treatment patterns in the ANCA associated Vasculitis market, which will assist our clients in analyzing the overall epidemiology and market scenario.
Some expert opinions have been provided below:
Qualitative Analysis
We perform Qualitative and Market Intelligence analysis using various approaches, such as SWOT analysis and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the Analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving treatment landscape.
Conjoint Analysis analyzes multiple approved and emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, designation, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy. In efficacy, the trial’s primary and secondary outcome measures are evaluated. Based on these, the overall efficacy is evaluated.
Further, the therapies’ safety is evaluated wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the route of administration, order of entry and designation, probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Related Report @ Granulomatosis with Polyangiitis Market
ANCA associated Vasculitis Market Access and Reimbursement
Because newly authorized drugs are often expensive, some patients escape receiving proper treatment or use off-label, less expensive prescriptions. Reimbursement plays a critical role in how innovative treatments can enter the market. The cost of the medicine, compared to the benefit it provides to patients who are being treated, sometimes determines whether or not it will be reimbursed. Regulatory status, target population size, the setting of treatment, unmet needs, the number of incremental benefit claims, and prices can all affect market access and reimbursement possibilities.
The report further provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of approved therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
ANCA associated Vasculitis Market Report Insights
- ANCA associated Vasculitis Patient Population
- ANCA associated Vasculitis Therapeutic Approaches
- ANCA associated Vasculitis Market Size
- ANCA associated Vasculitis Market Trends
- Existing ANCA associated Vasculitis Market Opportunity
ANCA associated Vasculitis Market Report Key Strengths
- Eleven-year Forecast
- The 7MM Coverage
- ANCA associated Vasculitis Epidemiology Segmentation
- Key Cross Competition
ANCA associated Vasculitis Market Report Assessment
- Current ANCA associated Vasculitis Treatment Practices
- Reimbursements
- ANCA associated Vasculitis Market Attractiveness
- Qualitative Analysis (SWOT, Conjoint Analysis, Unmet needs)
Key Questions
- Would there be any changes observed in the current treatment approach?
- Will there be any improvements in ANCA associated Vasculitis management recommendations?
- Would research and development advances pave the way for future tests and therapies for ANCA associated Vasculitis?
- Would the diagnostic testing space experience a significant impact and lead to a positive shift in the treatment landscape of ANCA associated Vasculitis?
- What kind of uptake will the new therapies witness in coming years in ANCA associated Vasculitis patients?
Explore our assortment of related Articles, Video and infographics to spark new ideas and inspiration:-
- ANCA-associated Vasculitis Treatment Market: Unraveling the Complexities
- ANCA Associated Vasculitis Market Infographics
- ANCA Vasculitis Market: Video
Stay updated with our Latest Articles @ Latest DelveInsight Blog

-in-the-7MM.jpg)
.jpg)

.jpg)