BCMA Targeted Therapies Market Summary
- The B Cell Maturation Antigen (BCMA) Targeted Therapies market in the 7MM is projected to grow at a significant CAGR by 2034 in leading countries (US, EU4, UK and Japan).
B Cell Maturation Antigen (BCMA) Targeted Therapies Market and Epidemiology Analysis
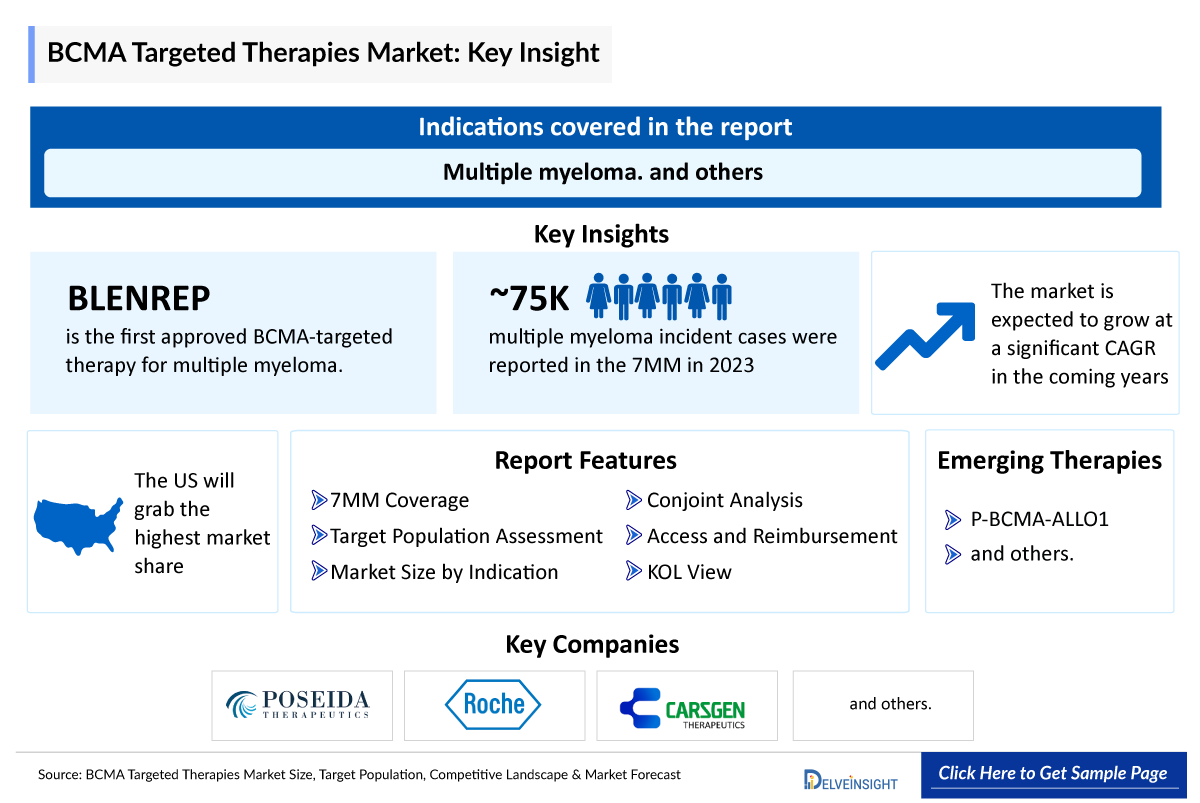
- Upon approval in August 2020, BLENREP (belantamab mafodotin), an antibody-drug conjugate, became the first drug to get approved as a B-cell maturation antigen (BCMA) targeted therapy for multiple myeloma. It was developed by GlaxoSmithKline (GSK), but in 2023 it was withdrawn from the US market after failing a late-stage study comparing its efficacy to existing treatments.
- Two Bi-specific T-cell engagers, Johnson & Johnson’s TECVAYLI and Pfizer’s ELREXFIO, were approved by the FDA in 2022 and 2023, respectively, for the treatment of adult patients with relapsed or refractory multiple myeloma who have received at least four prior lines of therapy.
- The total incidence of Multiple Myeloma in the 7MM was nearly 75,000 in 2023 and is expected to rise during the forecast period.
- Currently, Various BCMA Targeted therapies such as TECVAYLI, CARVYKTI, ELREXFIO, ABECMA and others are approved by the FDA for the treatment of relapsed or refractory multiple myeloma.
- BCMA, a protein found in the tumor necrosis factor receptor family, is crucial for the growth and survival of myeloma cells. Its presence primarily on malignant plasma cells makes it a promising target for treating multiple myeloma. Several therapies targeting BCMA, including antibody-drug conjugates (ADCs), chimeric antigen receptor (CAR)-T cells, and bispecific T cell engagers, have shown significant effectiveness in patients with relapsed and refractory multiple myeloma
- Moreover, several BCMA targeted therapies are currently being evaluated in clinical trials. An example is CARsgen therapeutic’s, Zevor-cel/zevorcabtagene autoleucel (CT053) which is in the developmental stage and is anticipated to receive approval during the forecast period (2024-2034).
- In February 2024, AstraZeneca finalized its acquisition of Gracell Biotechnologie for around USD 1.2 billion, enhancing its portfolio with GC012F, a pioneering FasTCAR-enabled BCMA therapy with potential applications in multiple myeloma, other blood cancers, and autoimmune conditions such as systemic lupus erythematosus (SLE).
- In March 2024, FDA granted orphan drug designation to P-BCMA-ALLO1, a novel BCMA-targeted allogeneic, T stem cell memory (TSCM)-rich chimeric antigen receptor (CAR) T-cell therapy for the treatment of relapsed/refractory multiple myeloma.
- As of April 2024, CARVYKTI is the first and only BCMA-targeted treatment approved by the FDA for patients with relapsed or refractory multiple myeloma who have received at least one prior line of therapy. Approval is based on results from the Phase 3 CARTITUDE-4 study, in which treatment with CARVYKTI in 1-3 prior lines of therapy reduced the risk of disease progression or death by 59 % compared to standard therapies
- On April 2024, BMS announced that the FDA has approved ABECMA (idecabtagene vicleucel) for the treatment of adult patients with relapsed or refractory multiple myeloma after two or more prior lines of therapy including an immunomodulatory agent (IMiD), a proteasome inhibitor (PI), and an anti-CD38 monoclonal antibody. ABECMA is a CAR T cell therapy that recognizes and binds to B-cell maturation antigen on the surface of multiple myeloma cells leading to CAR T cell proliferation, cytokine secretion, and subsequent cytolytic killing of BCMA-expressing cells.
- ABECMA was jointly developed and commercialized in the United States as part of a co-development, co-promotion, and profit share agreement between BMS and 2seventy bio, and was originally approved by the FDA in March 2021 for the treatment of adult patients with relapsed or refractory multiple myeloma after four or more prior lines of therapy.
- Poseida therapeutics/ Roche, CARsgen and several other companies are currently engaged in the development and production of BCMA targeted , which has the potential to significantly impact and enhance the BCMA targeted therapies market.
Request for unlocking the Sample Page of the BCMA Targeted Therapies Market
DelveInsight’s “B Cell Maturation Antigen (BCMA) Targeted Therapies” Target Population, Competitive Landscape, and Market Forecast – 2034” report delivers an in-depth understanding of the BCMA targeted therapies, historical and forecasted epidemiology, competitive landscape as well as the BCMA targeted therapies market trends in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
The BCMA Targeted Therapies Market report provides current treatment practices, emerging drugs, market share of individual therapies, and current and forecasted 7MM BCMA targeted therapies market size from 2020 to 2034. The BCMA targeted therapies market report also covers current BCMA targeted therapies treatment practices/algorithms and unmet medical needs to curate the best opportunities and assess the market’s potential.
|
Study Period |
2020 to 2034 |
|
Geographies Covered |
|
|
BCMA Targeted Therapies Companies |
|
BCMA Targeted Therapies Treatment Market
B-cell maturation antigen (BCMA), is a protein which is the member of the tumor necrosis factor receptor family. It is involved in myeloma cell growth and survival, on the surface of their myeloma cells. BCMA is involved in regulating B cell maturation and survival. It interacts with a protein called APRIL (a proliferation-inducing ligand) to promote the survival of plasma cells. BCMA is primarily expressed on the surface of malignant multiple myeloma B-lineage cells, as well as late-stage B cells and plasma cells.
BCMA Targeted Therapies Treatment
B cell maturation antigen (BCMA) is a novel treatment target for multiple myeloma due to its highly selective expression in malignant plasma cells. Multiple BCMA-targeted therapeutics, including antibody-drug conjugates (ADC), chimeric antigen receptor (CAR)-T cells, and bispecific T cell engagers, have achieved remarkable clinical response in patients with relapsed and refractory multiple myeloma. Antibody-drug conjugates consist of a monoclonal antibody targeting BCMA linked to a cytotoxic drug. Upon binding to BCMA-expressing myeloma cells, the ADC is internalized, releasing the cytotoxic drug and causing cell death.
Chimeric antigen receptor (CAR) T-cell Therapy is involved in modifying a patient's own T cells to express a chimeric antigen receptor that targets BCMA. These engineered T cells can recognize and kill myeloma cells expressing BCMA when infused back into the patient. Bispecific T-cell engager antibodies are designed to simultaneously bind to BCMA on myeloma cells and CD3 on T cells. This brings the T cells in close proximity to the myeloma cells, leading to their activation and killing. Monoclonal Antibodies: Monoclonal antibodies (mAbs) targeting BCMA, such as belantamab mafodotin, bind specifically to BCMA on the surface of myeloma cells. This binding can trigger various mechanisms leading to the destruction of myeloma cells, including antibody-dependent cellular cytotoxicity (ADCC) and antibody-dependent cellular phagocytosis (ADCP).
BCMA Targeted Therapy Drug Chapters
The drug chapter segment of the BCMA targeted therapies reports encloses a detailed analysis of BCMA targeted therapies marketed drugs and late-stage (Phase II and Phase I) pipeline drugs. It also helps understand the BCMA targeted therapies' clinical trial details, expressive pharmacological action, agreements and collaborations, approval and patent details, advantages and disadvantages of each included drug and the latest news and press releases.
Marketed BCMA Targeted Therapies Drugs
- TECVAYLI (teclistamab-cqyv): Janssen Biotech
TECVAYLI is a first-in-class, bispecific T-cell engager antibody that is administered as a subcutaneous treatment. This off-the-shelf (or ready to use) therapy activates the immune system by binding to the CD3 receptor expressed on the surface of T-cells and to the B-cell maturation antigen (BCMA) expressed on the surface of multiple myeloma cells and some healthy B-lineage cells. TECVAYLI (teclistamab-cqyv) is approved for the treatment of adult patients with relapsed or refractory multiple myeloma, who previously received four or more prior lines of therapy, including a proteasome inhibitor, immunomodulatory drug and anti-CD38 monoclonal antibody. TECVAYL, an off-the-shelf, subcutaneous therapy, is an important new medicine for patients with incurable blood cancer who face limited treatment options.
|
Product |
Company |
Indication |
|
TECVAYLI (Teclistamab-cqyv) |
Janssen biotech |
Treatment of adult patients with relapsed or refractory multiple myeloma who have received at least four prior lines of therapy, including a proteasome inhibitor, an immunomodulatory agent and an anti-CD38 monoclonal antibody |
Note: Detailed current therapies assessment will be provided in the full report of BCMA targeted therapies.
Emerging BCMA Targeted Therapies Drugs
- Zevor-cel (CT053): CARsgen Therapeutics
Zevor-cel (CT053) is a fully human, single autologous chimeric B-cell maturation antigen (BCMA) CAR T-cell product candidate tested for the treatment of multiple myeloma.
Zevor-cel received regenerative medicine advanced therapy (RMAT) and orphan drug designations from the FDA in 2019, as well as the priority medicine (PRIME) and orphan medicinal product designations from the european medicines agency (EMA) in 2019 and 2020, respectively. Zevor-cel also received breakthrough therapy designation from the NMPA in 2020.
- P-BCMA-ALLO1: Poseida therapeutics/ Roche
This allogeneic CAR-T candidate targeting B cell maturation antigen, or BCMA, is in development to treat relapsed/refractory multiple myeloma. It offers an improved VH-based binder which targets BCMA. It is being developed in partnership with Roche and has shown early evidence of encouraging safety and efficacy.
|
List of Emerging Drugs | |||||
|
Zevor-cel/ zevorcabtagene autoleucel (CT053) |
CARsgen Therapeutics |
Multiple Myeloma |
BCMA targeted therapy |
I/II |
NCT03915184 |
|
P-BCMA-ALLO1 |
Poseida therapeutics/ Roche |
Multiple Myeloma |
BCMA targeted therapy |
I |
NCT04960579 |
BCMA Targeted Therapy Market Outlook
The BCMA Targeted Therapies Market is expected to grow significantly in the coming years. This is due to the increasing number of patients who are being diagnosed with cancer, the growing awareness of BCMA targeted therapies, the increasing number of BCMA targeted therapies that are under clinical trials, and the increasing interest of major pharmaceutical companies toward it.
The first drug approved as a B-cell maturation antigen BCMA targeted therapy for the treatment of multiple myeloma was BLENREP (belantamab mafodotin). It was developed by GlaxoSmithKline (GSK) and was granted accelerated approval by the FDA in August 2020 for the treatment of adult patients with relapsed or refractory multiple myeloma who have received at least four prior therapies, including an anti-CD38 monoclonal antibody, a proteasome inhibitor, and an immunomodulatory agent but it was withdrawn from the United states markets in 2023 as it failed a late-stage study designed to show it was better than an existing treatment available in the market.
On April 2024, BMS announced that the FDA has approved ABECMA (idecabtagene vicleucel) for the treatment of adult patients with relapsed or refractory multiple myeloma after two or more prior lines of therapy including an immunomodulatory agent (IMiD), a proteasome inhibitor (PI), and an anti-CD38 monoclonal antibody. ABECMA is a CAR T cell therapy that recognizes and binds to B-cell maturation antigen on the surface of multiple myeloma cells leading to CAR T cell proliferation, cytokine secretion, and subsequent cytolytic killing of BCMA-expressing cells. The product was jointly developed and commercialized in the United States as part of a co-development, co-promotion, and profit share agreement between BMS and 2seventy bio, and was originally approved by the FDA in March 2021 for the treatment of adult patients with relapsed or refractory multiple myeloma after four or more prior lines of therapy.
Currently, Various BCMA Targeted therapies such as TECVAYLI, CARVYKTI, ELREXFIO, ABECMA and others are approved by the US FDA. Several key BCMA Targeted Therapies Companies, including Poseida therapeutics/ Roche, CARsgen Therapeutics and others, are involved in developing drugs for BCMA targeted therapies for indications such as multiple myeloma, and others. Overall, this is an exciting new class of agents with great potential for development. The maturation of current studies over the next few years will lead to a better understanding of BCMA targeted therapies and define their role in the therapy of cancer.
BCMA Targeted Therapies Drug Uptake
This section focuses on the uptake rate of potential approved and emerging BCMA targeted therapies expected to be launched in the BCMA Targeted Therapies market during 2020–2034.
BCMA Targeted Therapies Pipeline Development Activities
The BCMA Targeted Therapies market forecast report provides insights into different therapeutic candidates in Phase II, and Phase I. It also analyzes key BCMA Targeted Therapies Companies involved in developing targeted therapeutics. The presence of numerous drugs under different stages is expected to generate immense opportunity for BCMA targeted therapies market growth over the forecasted period.
Pipeline Development Activities
The BCMA Targeted Therapies market forecast report covers information on collaborations, acquisitions and mergers, licensing, and patent details for emerging BCMA targeted therapies.
Recent Key Events in the BCMA Targeted Therapies Market
The increasing strategic collaborations among major players to enhance the growth of their pipeline products are anticipated to drive market expansion.
- In February 2024, Astrazeneca completed the acquisition of Gracell Biotechnologie, a global clinical-stage biopharmaceutical company developing innovative cell therapies for the treatment of cancer and autoimmune diseases for approximately USD 1.2 billion.
- The acquisition enriches AstraZeneca’s growing pipeline of cell therapies with GC012F, a novel, clinical-stage FasTCAR-enabled BCMA. GC012F is a potential new treatment for multiple myeloma, as well as other haematologic malignancies and autoimmune diseases including systemic lupus erythematosus (SLE).
Latest KOL Views on BCMA Targeted Therapies
To keep up with current and future BCMA Targeted Therapies market trends, we take Industry Experts’ opinions working in the domain through primary research to fill the data gaps and validate our secondary research. Industry experts were contacted for insights on BCMA targeted therapies' evolving BCMA Targeted Therapies market landscape, patient reliance on conventional therapies, patient therapy switching acceptability, drug uptake, along challenges related to accessibility.
DelveInsight’s analysts connected with 25+ KOLs to gather insights; however, interviews were conducted with 10+ KOLs in the 7MM. Their opinion helps understand and validate current and emerging therapy treatment patterns or BCMA targeted therapy inhibitors market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the BCMA Targeted Therapies market and the unmet needs.
Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving treatment landscape.
BCMA Targeted Therapies Market Access and Reimbursement
Reimbursement for BCMA targeted therapy has been universal in the United States and even in Europe. In the UK, as of february 2023, NICE was unable to make a recommendation on teclistamab (Tecvayli) for treating relapsed or refractory multiple myeloma after 3 or more therapies in adults. This is because agios did not provide an evidence submission.
Also as of May 2023, NICE was unable to make a recommendation on ciltacabtagene autoleucel (Carvykti) for treating relapsed or refractory multiple myeloma in adults. after 3 or more therapies in adults. This is because Janssen withdrew its evidence submission for the appraisal. The BCMA Targeted Therapies market forecast report further provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of approved therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Key Updates on BCMA targeted therapies
Many Pharma BCMA Targeted Therapies Companies will be presenting the data of their BCMA targeted therapy during the ASCO 2024 conference including Abbvie and others. During the ASCO 2024 annual meeting, Abbvie pharma is expected to present the results of clinical trial NCT03933735, with the title “Efficacy, safety, and determination of RP2D of ABBV-383, a BCMA bispecific antibody, in patients with relapsed/refractory multiple myeloma (RRMM)” (Abstract # 7531).
The abstract list is not exhaustive, will be provided in the final report
BCMA Targeted Therapies Market Report Scope
- The BCMA Targeted Therapies market forecast report covers a segment of key events, an executive summary, and a descriptive overview, explaining its mechanism, and therapies (current and emerging).
- Comprehensive insight into the Competitive Landscape, and forecasts, the future growth potential of treatment rate, drug uptake, and drug information have been provided.
- Additionally, an all-inclusive account of the current and emerging therapies and the elaborative profiles of late-stage and prominent therapies will impact the current landscape.
- A detailed review of the BCMA targeted therapies market forecast, historical and forecasted BCMA Targeted Therapies market size, BCMA Targeted Therapies market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The BCMA Targeted Therapies market report provides an edge while developing business strategies, by understanding trends, through SWOT analysis expert insights/KOL views, and treatment preferences that help shape and drive the 7MM BCMA targeted therapies market.
BCMA Targeted Therapies Market Report Insights
- BCMA Targeted Therapies Targeted Patient Pool
- BCMA Targeted Therapies Therapeutic Approaches
- BCMA Targeted Therapies Pipeline Analysis
- BCMA Targeted Therapies Market Size
- BCMA Targeted Therapies Market Trends
- Existing and Future BCMA Targeted Therapies Market Opportunity
BCMA Targeted Therapies Market Report Key Strengths
- 10 years BCMA Targeted Therapies Market Forecast
- The 7MM Coverage
- Key Cross Competition
- BCMA Drugs Uptake
- Key BCMA Targeted Therapies Market Forecast Assumptions
BCMA Targeted Therapies Market Forecast Report Assessment
- Current BCMA Targeted Therapies Treatment Market Practices
- BCMA Targeted Therapies Unmet Needs
- BCMA Targeted Therapies Pipeline Product Profiles
- BCMA Targeted Therapies Market Attractiveness
- Qualitative Analysis (SWOT)
- BCMA Targeted Therapies Market Drivers
- BCMA Targeted Therapies Market Barriers
Key Questions Answered In The BCMA Targeted Therapies In The Market Report
- What was the BCMA targeted therapies market size, the BCMA Targeted Therapies market size by therapies, BCMA Targeted Therapies market share (%) distribution, and what would it look like in 2034? What are the contributing factors for this growth?
- Which drug is going to be the largest contributor in 2034?
- Which is the most lucrative BCMA targeted therapies Market?
- Which drug type segment accounts for maximum BCMA targeted therapies sales?
- What are the pricing variations among different geographies for approved therapies?
- How has the reimbursement landscape for BCMA targeted therapies evolved since the first one was approved? Do patients have any access issues that are driven by reimbursement decisions?
- What are the risks, burdens, and unmet needs of treatment with BCMA targeted therapies? What will be the growth opportunities across the 7MM for the patient population on BCMA targeted therapies?
- What are the key factors hampering the growth of the BCMA targeted therapies market?
- What are the indications for which recent novel therapies and technologies have been developed to overcome the limitations of existing treatments?
- What key designations have been granted to the therapies for BCMA targeted therapies?
- What is the cost burden of approved BCMA Targeted Therapies on the patient?
- Patient acceptability in terms of preferred therapy options as per real-world scenarios?
- What are the country-specific accessibility issues of expensive, recently approved BCMA Targeted Therapies?
Reasons to buy the BCMA Targeted Therapies Market Forecast Report
- The BCMA Targeted Therapies market size report will help develop business strategies by understanding the latest trends and changing dynamics driving the BCMA targeted therapies Market.
- Understand the existing BCMA Targeted Therapies market opportunities in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain) the United Kingdom, and Japan.
- Identifying strong upcoming BCMA companies in the BCMA Targeted Therapies market will help devise strategies to help get ahead of competitors.
- Detailed analysis and ranking of indication-wise current and emerging therapies under the conjoint analysis section to provide visibility around leading indications.
- Highlights of Access and Reimbursement policies of approved therapies, barriers to accessibility of expensive off-label therapies, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing BCMA Targeted Therapies market so that the upcoming BCMA Targeted Therapies manufacturers can strengthen their development and launch strategy.
Stay Updated with us for Recent Articles on the BCMA Targeted Therapies
- Clinical activity of P-BCMA-ALLO1, a B-cell maturation antigen (BCMA) targeted allogeneic chimeric antigen receptor T-cell (CAR-T) therapy, in relapsed refractory multiple myeloma (RRMM) patients (pts) following progression on prior BCMA targeting therapy
- Merck supports Peloton Therapeutics; Thermo Fisher, WuXi and Mayo Clinic join hands; JNJ's anti-BCMA CAR-T sweeps multiple myeloma aside; Tafinlar-Mekinist combo of Novartis receives a boost
- Latest DelveInsight Blogs