Brain Cancer Market
- The largest Brain Cancer market size in the 7MM was occupied by the US in 2023.
- Among EU4 and the UK, Italy will capture the maximum revenue share, followed by Germany in 2034.
- The US contributed to the largest incident-patient share, acquiring more than 38% of the 7MM in 2023.
- Among the gender-specific cases, males are affected more than females, accounting for nearly 60% and 40% of cases respectively, in the US, in 2023.
- Among the grade-specific cases, the cases of high-grade (III and IV) brain tumors are more incident than Low-Grade, accounting for nearly 70% of cases in the US.
- In April 2024, the United States Food and Drug Administration (US FDA) granted accelerated approval to Day One Biopharmaceuticals’ OJEMDA (tovorafenib) as a treatment for patients 6 months of age and older with certain relapsed or refractory pediatric low-grade glioma.
- In April 2024, TME Pharma announced that the US FDA granted Fast Track Designation for NOX-A12 (olaptesed pegol), TME Pharma's CXCL12 inhibitor, in combination with radiotherapy and bevacizumab for use in the treatment of the aggressive adult brain cancer, glioblastoma.
- In February 2024, Servier announced that the US FDA accepted the filing and granted priority review for the New Drug Application (NDA) for vorasidenib, and assigned a Prescription Drug User Fee Act (PDUFA) action date of August 20, 2024. If approved, vorasidenib would become a first-in-class targeted therapy for patients with IDH-mutant gliomas.
Request for unlocking the Sample Page of the Brain Tumor Diagnosis Treatments Market
Factors affecting Brain Cancer Market Growth
-
Rising Incidence of Brain Tumors
The increasing prevalence of primary and metastatic brain tumors worldwide is a significant driver for the brain cancer market. Early detection and advanced treatment options are becoming more critical due to the growing patient population.
-
Advancements in Diagnostic Techniques
Innovations in imaging technologies such as MRI, PET scans, and molecular diagnostics enable earlier and more accurate detection of brain cancer. These advancements enhance treatment planning and improve patient outcomes, driving market growth.
-
Emergence of Targeted Therapies and Immunotherapies
The development of targeted therapies, immunotherapies, and personalized treatment approaches has expanded treatment options for brain cancer patients. These novel therapies increase survival rates and encourage market adoption.
-
Increasing Geriatric Population
Age is a major risk factor for many types of brain cancers. With the global population aging, there is a higher prevalence of brain tumors, contributing to increased demand for effective therapies and management solutions.
-
Rising Awareness and Screening Programs
Growing awareness about brain cancer symptoms and the importance of early diagnosis has led to more screenings and timely medical intervention, supporting market expansion.
-
Government Initiatives and Funding for Cancer Research
Increased funding and government-led initiatives for cancer research and treatment development have accelerated the availability of advanced therapies, boosting market growth.
-
Expansion of Healthcare Infrastructure in Emerging Markets
Improved access to oncology care, advanced diagnostic facilities, and treatment centers in emerging regions is increasing patient reach and driving market adoption.
DelveInsight’s "Brain Cancer Market Insight, Epidemiology, and Market Forecast – 2034" report delivers an in-depth understanding of brain cancer, historical and forecasted epidemiology as well as the brain cancer market trends in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
The Brain Tumor Diagnostics Market Report provides current treatment practices, emerging drugs, Brain cancer market share of individual therapies, and current and forecasted Brain cancer market size from 2020 to 2034, segmented by seven major markets. The report also covers current Brain cancer treatment practices/algorithms and unmet medical needs to curate the best of the opportunities and assess the underlying potential of the market.
|
Study Period |
2020 to 2034 |
|
Forecast Period |
2024-2034 |
|
Geographies Covered |
|
|
Brain Tumor Market |
|
|
Brain Tumor Market Size | |
|
Brain Tumor Companies |
|
Brain Cancer Treatment Market Understanding
A brain tumor, known as an intracranial tumor, central nervous system (CNS) tumors represent a group of diseases that have in common the abnormal development of mass lesions in the brain, spinal cord, or its coverings. A brain tumor can be classified into two main groups, i.e., primary and metastatic. A primary brain tumor is often described as “low grade” or “high grade.” A low-grade tumor generally grows slowly, but it can turn into a high-grade tumor, whereas a high-grade tumor is more likely to grow faster. Secondary brain tumors, also called brain metastases, are much more common than primary tumors in adults.
Brain Cancer Diagnosis
Most brain tumors are not diagnosed until after symptoms appear. In general, diagnosing a brain tumor usually begins with magnetic resonance imaging (MRI). Once an MRI shows a tumor in the brain, the most common way to determine the type of brain tumor is to look at the results from a tissue sample after a biopsy or surgery. Blood tests are also done to help diagnose certain brain tumors such as pituitary gland, pineal region, and germ cell tumors. Sophisticated imaging techniques are used to pinpoint brain tumors. Diagnostic tools used for this purpose include computed tomography (CT or CAT scan) and MRI. Other MRI sequences can help the surgeon plan the resection of the tumor based on the location of the normal nerve pathways of the brain. Intraoperative MRI is also used during surgery to guide tissue biopsies and tumor removal.
Further details related to diagnosis will be provided in the report...
Brain Cancer Treatment
Brain Tumor Treatment options for brain cancers include surgery, radiation therapy, chemotherapy, and targeted therapy. The treatment options and recommendations depend on several factors like the size, type, and grade of the tumor, whether the tumor is putting pressure on vital parts of the brain if the tumor has spread to other parts of the CNS or the body, the possible side effects and finally, the treatment preferences and overall health of the patient. For a low-grade brain tumor, surgery may be the only treatment needed, especially if all of the tumor can be removed. If there is a visible tumor remaining after surgery, radiation therapy and chemotherapy may be used. For higher-grade tumors, treatment usually begins with surgery, followed by radiation therapy and chemotherapy.
Further details related to treatment will be provided in the report...
Brain cancer Epidemiology
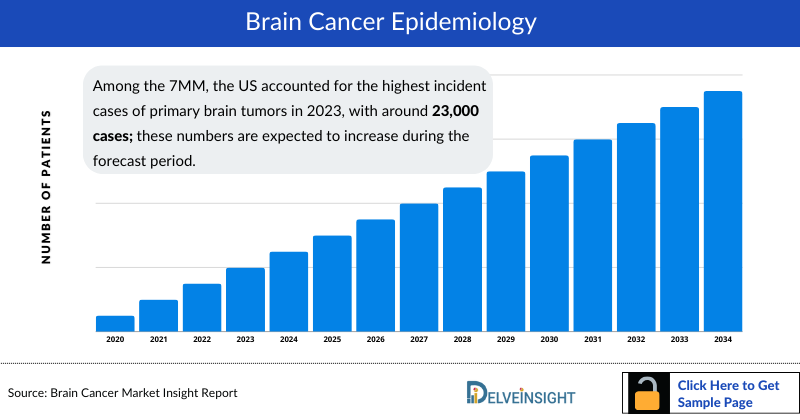
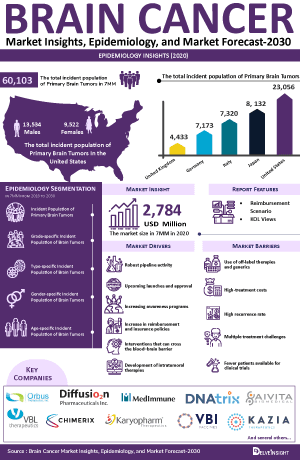
The brain cancer epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by the Total incident cases of Primary Brain Tumors, Type-specific incident cases of Brain Tumor, Gender-specific incident cases of Brain Tumor, Grade-specific incident cases of Brain Tumor, and Age-specific Distribution of Brain Tumor in the 7MM covering the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan from 2020 to 2034.
- Among the 7MM, the US accounted for the highest incident cases of primary brain tumors in 2023, with around 23,000 cases; these numbers are expected to increase during the forecast period.
- According to the estimates, in the US, it is observed that brain tumor was most incident in the 40-64 years age group, followed by ≥65 years.
- Among the type-specific cases, the cases of Glioblastoma Multiforme accounted for the highest number of incident cases in 2023 in the US.
- Amongst EU4 and the UK, Italy accounted for the highest number of incident cases of the primary brain tumor, followed by Germany in 2023.
Unlock comprehensive insights! Click Here to Purchase the Full Epidemiology Report @ Brain Cancer Prevalence
Brain Cancer Market Recent Developments and Breakthroughs
- In January 2025, the FDA granted breakthrough device designation to CergenX’s AI-powered tool, Wave, for neonatal brain scans. The Irish startup will receive prioritized review and enhanced communication with FDA experts during the premarket review phase.
- In December 2024, Kazia Therapeutics' stock dropped 26% to $2.28 after the FDA indicated it might consider standard approval, but not accelerated approval, for the company's brain cancer drug, paxalisib. The stock has fallen about 48% this year. Paxalisib, designed to treat glioblastoma, showed significant improvement in overall survival in a July trial, which previously led to a surge in the company's stock.
Brain Cancer Drug Chapters
The drug chapter segment of the Brain Tumor Diagnostics Market Report encloses a detailed analysis of the marketed and late-stage (Phase III) Brain Cancer pipeline drugs. The marketed drugs segment encloses drugs such as AVASTIN (Genentech), TEMODAR (Merck), GLIADEL Wafer (Azurity Pharmaceuticals), and others. Furthermore, the current key Brain Cancer Companies for emerging drugs and their respective drug candidates include Servier (Vorasidenib), Kazia Therapeutics (paxalisib), and others. The drug chapter also helps understand the brain cancer clinical trial details, expressive pharmacological action, agreements and collaborations, approval and patent details, and the latest news and press releases.
Brain Cancer Marketed Drugs
-
AVASTIN (bevacizumab): Genentech
AVASTIN is a recombinant humanized monoclonal IgG1 antibody, which acts as an angiogenesis inhibitor by blocking its target, vascular endothelial growth factor. It binds to the vascular endothelial growth factor with its receptors VEGFR-1 and VEGFR-2, which are present on the surface of endothelial cells. This helps in reducing the activity of VEGF and regressing the vascularization of tumors. AVASTIN is indicated for the treatment of glioblastoma with progressive disease in adult patients following prior therapy as a single agent. In December 2017, the US FDA granted full approval of AVASTIN for the treatment of adults with recurrent glioblastoma that has progressed following prior therapy. It was previously granted provisional approval in this setting under the US FDA’s accelerated approval program.
-
TEMODAR/TEMODAL: Merck
The active pharmaceutical ingredient in TEMODAR/TEMODAL is an imidazotetrazine derivative of the alkylating agent dacarbazine. It is used for the treatment of several brain cancer forms, e.g., as a second-line treatment for astrocytoma and as a first-line treatment for glioblastoma. The therapeutic benefit is due to its ability to alkylate/methylate DNA. This alkylation/methylation destroys the DNA and triggers the death of the tumor cells. Temozolomide targets selectively tumoral tissues; it has an anti-neoplastic effect; it has minimum influence on adjacent brain tissues; it has no severe systemic toxicity, and it is eliminated rapidly. It was granted US FDA approval in the treatment of recurrent anaplastic astrocytoma in 1999. Then, in March 2005, the US FDA approved TEMODAR for the treatment of adult patients with newly diagnosed glioblastoma multiforme concomitantly with radiotherapy and then as maintenance treatment. Recently, in September 2023, the US FDA approved new and updated indications for TEMODAR capsules and injections, including for the adjuvant treatment of adults with newly diagnosed anaplastic astrocytoma, and the treatment of adults with refractory anaplastic astrocytoma.
|
Comparison of Marketed Drugs | ||||
|
Product |
Company |
MoA |
RoA |
Approval Year |
|
AVASTIN |
Genentech |
VEGF inhibitor |
Intravenous |
2009 (US), 2013 (JP) |
|
TEMODAR/TEMODAL |
Merck |
Alkylation |
Oral |
1999 (US, EU), 2006 (JP) |
|
GLIADEL Wafer |
Azurity Pharmaceuticals |
Alkylation |
Intracranial Implant |
1996 (US), 1999 (EU), 2012 (JP) |
Brain Cancer Emerging Drugs
-
Vorasidenib: Servier
Vorasidenib is a small molecule and is a dual inhibitor of mutant isocitrate dehydrogenase 1 and 2 (IDH1/2) enzymes in development for the treatment of IDH-mutant diffuse glioma. Vorasidenib works as a brain penetrant. It was granted Fast Track Designation in February 2023 and Breakthrough Therapy Designation in August 2023 by the US FDA. In the Phase III INDIGO trial (NCT04164901), vorasidenib demonstrated reduced tumor growth rate (TGR) and shrunk tumor volume, as measured by an independent radiology committee, while patients in the placebo arm saw continued growth in tumor volume.
-
Paxalisib: Kazia Therapeutics
Paxalisib is a small molecule that inhibits PI3K, a critical control mechanism in growth and cell division, which is activated in many forms of cancer. Paxalisib has been designed to cross the blood-brain barrier, and a wealth of experimental data shows that it does so very successfully. This feature of paxalisib is almost unique in this class of medicines and differentiates it from the approved products in the PI3K inhibitor class. The lead indication for paxalisib is glioblastoma. In July 2023, the US FDA granted Fast Track Designation to paxalisib in combination with radiation therapy for the treatment of patients with solid tumor brain metastases harboring PI3K pathway mutations. The drug is currently in an adaptive phase III study for potential registration called GBM AGILE.
|
Comparison of Emerging Therapies | |||||
|
Emerging Drug |
Company |
Phase |
Molecule Type |
MoA |
RoA |
|
Vorasidenib |
Sevier |
III |
Small molecule |
IDH1 and 2 inhibitor |
Oral |
|
Paxalisib |
Kazia Therapeutics |
III |
Small molecule |
Inhibits the PI3K pathway |
Oral |
|
ONC201 |
Chimerix |
III |
Small molecule |
Highly selective antagonist of dopamine receptor D2 (DRD2) and ClpP agonist |
Oral |
|
AV GBM 1 |
Aivita Bio-medical |
III |
Dendritic cell vaccine |
Immuno-therapy |
Subcutaneous Injection |
|
MDNA55 |
Medicenna Therapeutics |
II |
Recombinant fusion protein |
Protein synthesis inhibitors |
Intratumoral |
Brain Tumor Therapeutics Market Class Insight
Apart from surgery and radiation therapy, chemotherapy and targeted therapy are often used for the treatment of brain cancer. Alkylating agents, another branch of chemotherapy, represent one of a few drug classes that embed the approved drug for brain cancer therapy. These drugs provide a cellular cytotoxic effect via crosslinking DNA molecules, causing double-strand breaks, hampering uncoiling, and leading to the apoptosis of a cell. Many drugs have entered clinical trials since 2010 as part of the alkylating agents class. Another prominent class of chemotherapy agents is topoisomerase inhibitors. Topoisomerases are vital for enzymes in human cells that regulate DNA supercoiling, thus providing cellular homeostasis during transcription and DNA replication. Few targeted therapies inhibit specific molecular targets involved in signaling pathways. A few common targets include EGFR, mTOR (mammalian target of rapamycin), PI3K (phosphatidylinositol 3-kinase), and VEGF (vascular endothelial growth factor). AVASTIN belongs to a class of drugs called VEGF inhibitors
Detailed drug class insight assessment will be provided in the final report...
Brain Cancer Market Outlook
The treatment often comprises a combination of several therapies, including surgery, chemotherapy, radiation, or stereotactic radiosurgery followed by additional/adjuvant treatments, such as chemotherapy or radiation therapy, after surgery. Treatment is palliative and may include surgery, radiation therapy, and/or chemotherapy. Immunotherapy provides another opportunity for treatment in the market. It is a new promising and exciting area of treatment designed to trigger the body’s immune system to fight and halt tumor growth. Immunotherapy or “vaccine” therapy involves the induction of an immune response against an individual tumor. The treatments might include checkpoint inhibitors and cancer vaccines that utilize a tumor’s antigens. The ongoing research lights up the chance for immunotherapy in the coming years. The other research alternatives include gene or oncolytic virus (polio or adeno or herpes virus) therapies as a way of controlling tumor growth. The number of companies working actively to develop therapeutic options for brain cancer is quite high in number. The go-ahead by the US FDA has put OJEMDA toe to toe with Novartis’ TAFINLAR-MEKINIST combo, which, thanks to an FDA approval last year, is allowed in pLGG but only for cases with BRAF V600 mutations. Multiple products from numerous companies like Genentech, Daiichi Sankyo, Bristol Myers Squibb, Bayer, Roche, Astra Zeneca, Pfizer, etc. are being developed clinically and tested in the late and mid-stage developments. Therefore, such companies hold a tremendous chance to capture the market share once their products are approved.
Key Findings
- Among the 7MM, the US accounted for the largest Brain Cancer Market Size in 2023, which is expected to grow during the forecast period (2024–2034).
- Among the current therapies, Temozolomide plus radiation therapy, or radiation therapy alone accounted for the largest market size in the US in 2023.
- Among EU4 and the UK, Italy accounted for the largest Brain Cancer market size, followed by Germany in 2023.
Brain Cancer Drugs Uptake
This section focuses on the uptake rate of potential Brain Cancer drugs expected to be launched in the market during 2024–2034. The brain cancer treatment market landscape has experienced a transformation with the uptake of novel drugs. These innovative therapies are redefining standards of care. Furthermore, the increased uptake of these transformative drugs is a testament to the unwavering dedication of Neurologists, professors of neurology, and director of the Neuromuscular Reference Center at the University Hospital, and professors in the Department of Translational Neuroscience. This momentous shift in treatment paradigms is a testament to the power of research, collaboration, and human resilience.
Brain Cancer Pipeline Development Activities
The Brain Tumor Treatment Market Report provides insights into therapeutic candidates in Phase III, Phase II, and Phase I/II. It also analyzes key Brain Cancer Companies involved in developing targeted therapeutics. Brain Cancer Companies like Merck, Daiichi Sankyo, Servier, and others are actively engaging their product in research and development efforts for brain cancer. The Brain Cancer pipeline possesses many potential drugs and there is a positive outlook for the therapeutics market, with expectations of growth during the forecast period (2024–2034).
Pipeline Development Activities
The Brain Tumor Diagnosis Treatments Market Report covers information on collaborations, acquisitions and mergers, licensing, and patent details for brain cancer emerging therapy.
Take Your Research to the Next Level! Click Here to Get Access to the Full Pipeline Report @ Brain Cancer Treatment Drugs
Latest KOL Views on Brain Cancer
To keep up with current market trends, we take KOLs and SMEs’ opinions working in the domain through primary research to fill the data gaps and validate our secondary research. Industry Experts contacted for insights on the brain cancer evolving Brain Cancer Treatment Market Landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake, along with challenges related to accessibility, including Neurology specialists, Neuroscience specialists, and others.
DelveInsight’s analysts connected with 30+ KOLs to gather insights; however, interviews were conducted with 15+ KOLs in the 7MM. Centers such as the University of Nebraska Medical Center, Centers for Disease Control and Prevention, Department of Translational Neuroscience, etc., were contacted. Their opinion helps understand and validate current and emerging therapy treatment patterns or brain cancer market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
Latest KOL Views on Brain Cancer Market
“GBM is the most aggressive form of brain cancer, often resulting in a patient’s death within one to two years from diagnosis. Historically, it is a very difficult disease to treat and current treatment options offer limited benefit to extend survival.” “A third line of research I would like to highlight is already in clinical trials and is the closest to reaching patients. We are repurposing a tubulin-targeting small molecule that prevents tubulin polymerization. It is an old antiparasitic drug called mebendazole, which is now being used in a Phase I dose escalation trial for newly diagnosed glioblastomas. We have improved the formulation of mebendazole and are working on other means of further improving the efficacy of the drug. It is surprisingly nontoxic and appears to be very effective in the laboratory.”
|
KOL Views |
|
“GBM is the most aggressive form of brain cancer, often resulting in a patient’s death within one to two years from diagnosis. Historically, it is a very difficult disease to treat and current treatment options offer limited benefit to extend survival.” |
|
“A third line of research I would like to highlight is already in clinical trials and is the closest to reaching patients. We are repurposing a tubulin-targeting small molecule that prevents tubulin polymerization. It is an old antiparasitic drug called mebendazole, which is now being used in a Phase I dose escalation trial for newly diagnosed glioblastomas. We have improved the formulation of mebendazole and are working on other means of further improving the efficacy of the drug. It is surprisingly nontoxic and appears to be very effective in the laboratory.” |
Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the Analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving treatment landscape.
Brain Tumor Market Access and Reimbursement
The treatment and management of brain tumors are expensive. A Brain Tumor represents a group of diseases that have abnormal development of mass lesions in the brain, spinal cord, or its coverings. The disease carries a high economic burden for patients and caregivers, much of which is associated with initial surgery. Some accelerated programs made available by the government to help drugs for glioblastoma, the most common type of brain cancer, reach the market. Novartis has a Universal Co-pay Program through which Patients may be eligible for immediate co-pay savings on their prescription. Eligible patients with private insurance may pay zero dollars per month, and Novartis would pay the remaining co-pay, up to USD 15,000 per calendar year, per product. The Musella Foundation For Brain Tumor Research & Information’s Brain Tumor Drug Copayment Assistance Program also provides financial assistance to families who need help covering the cost of certain drugs (AVASTIN, GLIADEL, OPTUNE, TEMODAR) used to treat primary malignant brain tumors (Grade 3 or 4).
Brain Tumor Diagnostics Market Report Scope
- The Brain Tumor Diagnostics Market Report covers a segment of key events, an executive summary, and a descriptive overview, explaining its causes, signs, symptoms, pathogenesis, and currently used therapies.
- Comprehensive insight into the Brain Tumor epidemiology segments and forecasts, disease progression, and treatment guidelines has been provided.
- Additionally, an all-inclusive account of the emerging therapies and the elaborative profiles of late-stage and prominent therapies will impact the current Brain Cancer Treatment Market Landscape.
- A detailed review of the Brain Tumor Diagnosis Treatments Market, historical and forecasted Brain Cancer market size, Brain Cancer market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The Brain Tumor Diagnosis Treatments Market Report provides an edge while developing business strategies by understanding trends through SWOT analysis and KOL views, patient journey, and treatment preferences that help shape and drive brain cancer.
Brain Tumor Diagnostics Market Report Insights
- Patient-based Brain Cancer Market Forecasting
- Therapeutic Approaches
- Brain Cancer Pipeline Analysis
- Brain Cancer Market Size and Trends
- Existing and Future Brain Tumor Diagnostics Market Opportunity
Brain Tumor Diagnosis Treatments Market Report Key Strengths
- 11 Years Brain Cancer Market Forecast
- The 7MM Coverage
- Brain Cancer Epidemiology Segmentation
- Key Cross Competition
- Brain Cancer Drugs Uptake
- Key Brain Cancer Market Forecast Assumptions
Brain Tumor Diagnostics Market Report Assessment
- Current Brain Cancer Treatment Market Practices
- Brain Cancer Unmet Needs
- Brain Cancer Pipeline Product Profiles
- Brain Tumor Diagnostics Market Attractiveness
- Qualitative Analysis (SWOT and Analyst Views)
FAQs
- What was the brain cancer market size, the Brain Cancer market size by therapies, Brain Cancer market share (%) distribution in 2020, and what would it look like by 2034? What are the contributing factors for this growth?
- What can be the future treatment paradigm for brain cancer?
- What are the disease risks, burdens, and unmet needs of brain cancer? What will be the growth opportunities across the 7MM concerning the patient population with brain cancer?
- What are the current options for the Brain Cancer treatment? What are the current guidelines for treating brain cancer in the 7MM?
- What are the recent novel therapies, targets, Brain Cancer mechanisms of action, and technologies being developed to overcome the limitations of existing therapies?
- What causes drug failure in brain cancer, and how many major players failed in 2023?
Reasons to Buy the Brain Tumor Diagnostics Market Report
- The Brain Tumor Diagnostics Market Report will help develop business strategies by understanding the latest trends and changing treatment dynamics driving the Brain Tumor Therapeutics Market.
- Insights on patient burden/disease Brain Cancer prevalence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years.
- Understand the existing Brain Tumor Diagnostics Market Opportunities in varying geographies and the growth potential over the coming years.
- Identifying strong upcoming players in the Brain Cancer therapeutics market will help devise strategies to help get ahead of competitors.
- Detailed analysis ranking of class-wise potential current and emerging therapies under the analyst view section to provide visibility around leading classes.
- Highlights of access and reimbursement policies of current therapies, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the Brain Cancer unmet needs of the existing market so that the upcoming players can strengthen their development and launch strategy
Stay Updated with us for Recent Articles
- Alpheus Medical Treats First High-Grade Glioma Brain Cancer Patient with its Proprietary Platform; Karidum’s Next Generation Globe® Pulsed Field System
- VistaGen’s PH94B for Anxiety Disorder; Keytruda for Head and Neck Cancer Treatment; Bavarian Nordic’s Smallpox Vaccine Imvanex; CAMP4 Raises USD 100 Million; Incyte's WU-CART-007; Incyte’s Opzelura for Vitiligo; AstraZeneca and Merck’s Lynparza; Sumitomo Pharma’s DSP-0390 for Brain Cancer
- The novel therapy for brain cancer treatments
- Identity Crises Faced by a Venerable Brain-Cancer Cell Line
- Latest DelveInsight Blogs