CAR T-Cell Therapy for Acute Lymphoblastic Leukemia Market
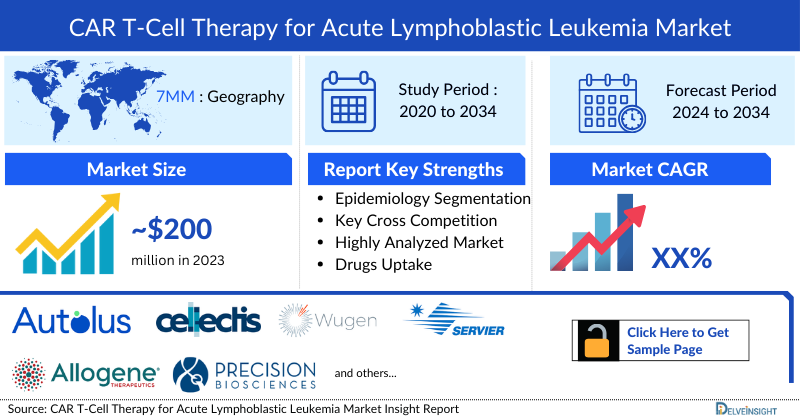
- In 2023, the CAR-T Cell Therapy for Acute Lymphoblastic Leukemia Market Size in the 7MM was approximately USD 200 million, which is further expected to increase by 2034
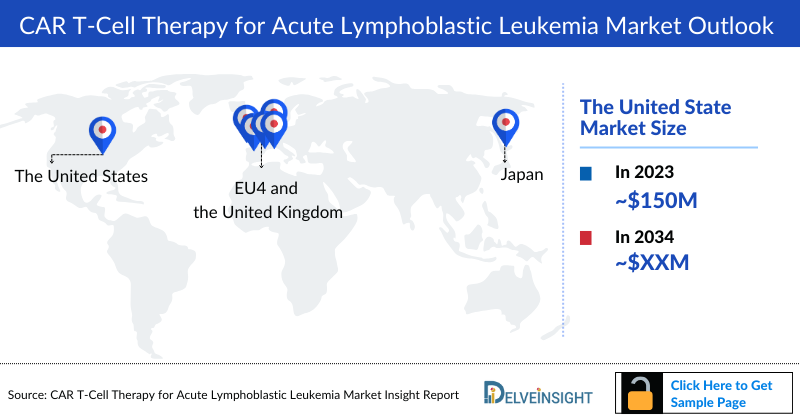
- In the 7MM, the US accounted for the largest CAR T-Cell Therapy for Acute Lymphoblastic Leukemia market size in 2023, with approximately USD 150 million.
- The main treatment options for Acute Lymphoblastic Leukemia include chemotherapy, targeted therapy, immunotherapy, surgery, radiation therapy, and stem cell transplant.
- In the last few years, immunotherapy has undergone a new phase of development which is linked to the development of CAR-T cell therapy. It is anticipated that CAR-T cells will bring out the next big leap forward in leukemia immunotherapy. In 2017, the US FDA made a historic decision by approving KYMRIAH (tisangenlecleucel), the first-ever CAR-T cell therapy for the treatment of acute lymphoblastic leukemia. In addition, later in October 2021, the FDA approved another CAR-T cell therapy, TECARTUS (brexucabtagene autoleucel) for adult patients 26 years of age and above with R/R B-cell precursor Acute Lymphoblastic Leukemia.
- CAR-T cells provide a long-term benefit with a one-time treatment, avoiding the toxicity of salvage chemotherapy and autologous transplant for patients with high-risk illnesses. The approvals have altered the standard of care for high-risk patients who are either primary refractory or have early recurrence following front-line therapy.
- Among the emerging CAR-Ts, obecabtagene autoleucel is anticipated to be the first to enter the market among the upcoming therapies, giving it a competitive edge over other emerging assets. Recently, in January 2024, the US FDA has accepted its BLA for obecabtagene autoleucel for patients with r/r Acute Lymphoblastic Leukemia. Under the Prescription Drug User Fee Act (PDUFA), the FDA has set a target action date of November 16, 2024.
- Although most clinical studies use autologous CAR-T cells for B-Acute Lymphoblastic Leukemia treatment, the administration of allogeneic CAR-T cells has been also reported in a small and limited number of clinical studies. Key players such as Precision Biosciences (PBCAR0191) Cellectis (UCART22), Wugen (WU-CART-007), and Cellectis/Servier/Allogene (UCART19) are currently testing their allogeneic products.
- CAR-T cell therapies need more data supporting their use in earlier lines of therapy. Patients are likely to have better outcomes if they receive CAR-T cell therapy before multiple lines of chemotherapy, like those in the clinical trials that led to FDA approval. Some trials are being designed to establish the efficacy and safety of CAR-T cell therapy in earlier lines. Novartis is expecting the submission schedule of KYMRIAH in first-line high-risk Acute Lymphoblastic Leukemia pediatrics and young adult patients in =2026.
Request for unlocking the Sample Page of the "CAR T-Cell Therapy for Acute Lymphoblastic Leukemia Market"
Factors affecting Car T Therapy For Acute Lymphoblastic Leukemia All Market Growth
-
Rising Incidence of Acute Lymphoblastic Leukemia
The growing prevalence of ALL, particularly in pediatric and adult populations, is a significant market driver. Patients with relapsed or refractory ALL have limited treatment options, increasing the demand for innovative therapies like CAR-T.
-
Advancements in CAR-T Therapy Technology
Continuous innovations in CAR-T cell engineering, including improved specificity, reduced toxicity, and enhanced persistence of engineered T-cells, are driving adoption and expanding the therapy’s market potential.
-
Regulatory Approvals and Clinical Advancements
The approval of CAR-T therapies for ALL by major regulatory bodies has boosted clinician confidence and patient access. Ongoing clinical trials are also exploring CAR-T in combination with other therapies, widening its therapeutic scope.
-
Unmet Medical Need in Relapsed/Refractory ALL
Traditional chemotherapy and bone marrow transplantation often fail in relapsed/refractory ALL cases. CAR-T therapy offers a novel and highly effective approach, driving market growth.
-
Rising Awareness and Acceptance of Immunotherapies
Increasing knowledge among healthcare providers and patients about immunotherapies’ benefits, especially CAR-T therapy’s targeted action and potential for long-term remission, supports market expansion.
-
Supportive Healthcare Infrastructure and Investment
Investments in specialized cell therapy centers, advanced laboratory facilities, and manufacturing capabilities improve accessibility and adoption of CAR-T therapies in ALL treatment.
-
Potential for Personalized Medicine
CAR-T therapy is inherently personalized, using a patient’s own T-cells to create tailored treatments. This approach aligns with the broader trend toward precision medicine, driving interest and uptake.
-
Favorable Reimbursement Policies
Increasing insurance coverage and government support for CAR-T therapies make these advanced treatments more accessible, particularly in developed markets, encouraging adoption.
DelveInsight’s “CAR-T Cell Therapy for Acute Lymphoblastic Leukemia Market Insight, Epidemiology and Market Forecast – 2034” report delivers an in-depth understanding of Acute Lymphoblastic Leukemia, historical and forecasted epidemiology as well as the CAR-T Cell Therapy for Acute Lymphoblastic Leukemia market trends in the United States, EU4 (Germany, France, Italy, and Spain), and the United Kingdom, and Japan.
The CAR-T Cell Therapy for Acute Lymphoblastic Leukemia Treatment Market Report provides current treatment practices, emerging CAR-Ts, market share of individual CAR-Ts, and current and forecasted 7MM CAR-T Cell Therapy for Acute Lymphoblastic Leukemia market size from 2020 to 2034. The report also covers current treatment practices/algorithms and unmet medical needs to curate the best opportunities and assess the market’s potential.
|
Study Period |
2020 to 2034 |
|
Forecast Period |
2024-2034 |
|
Geographies Covered |
|
|
CAR T-Cell Therapy for Acute Lymphoblastic Leukemia Market |
|
|
CAR T-Cell Therapy for Acute Lymphoblastic Leukemia Market Size | ~USD 200 Million in 2023 |
|
CAR T-Cell Therapy for Acute Lymphoblastic Leukemia Companies |
|
Key Factors Driving the CAR T-Cell Therapy for Acute Lymphoblastic Leukemia Market
Increase in ALL Incidence
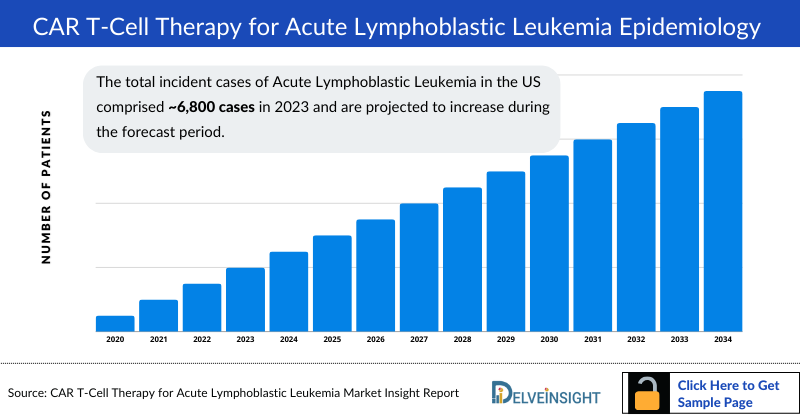
Among the 7MM, the US accounted for highest number of incident cases of ALL, i.e., approximately 6,900 cases in 2024. These numbers are further expected to increase by 2034.
Rise of Allogeneic Cell Therapy
The rise of allogeneic cell therapy within the CAR-T pipeline offers significant opportunities due to its potential for increased safety compared to autologous CAR-T, lower costs, and off-the-shelf availability.
Dual Targeting in CAR-T Therapy
Companies are leveraging the strength of dual targeting in CAR-T therapies in order to avoid the antigen escape that occurs in almost half of relapses that are caused by single antigen targeting CAR T-cells. Autolus has designed AUTO1/22 to target both CD19 and CD22.
Launch of Emerging CAR T-Cell Therapies for Acute Lymphoblastic Leukemia
Some of the therapies in the pipeline include WU-CART-007 (Wugen), AUTO1 (Autolus Therapeutics), UCART22 (Cellectis), PMB-101 (PeproMene Bio), FT819 (Fate Therapeutics), and others.
CAR-T Cell Therapy for Acute Lymphoblastic Leukemia Treatment Market
Acute lymphocytic leukemia, also known as acute lymphoblastic leukemia, is a type of cancer that affects the blood and bone marrow. It starts from young white blood cells called lymphocytes in the bone marrow; mainly characterized by an overproduction of immature white blood cells, called lymphoblasts or leukemic blasts. Because the bone marrow is unable to make adequate numbers of red cells, normal white cells, and platelets, people with Acute Lymphoblastic Leukemia become more susceptible to anemia, recurrent infections, and bruising and bleeding easily. The blast cells can then spill out of the bone marrow into the bloodstream and accumulate in various organs including the lymph nodes or glands, spleen, liver, and central nervous system (brain and spinal cord). Tests and procedures used to diagnose Acute Lymphoblastic Leukemia include blood tests, bone marrow tests, imaging tests, and spinal fluid tests.
The treatment options for Acute Lymphoblastic Leukemia include chemotherapy, post-remission therapy (consolidation and maintenance therapy), targeted therapy, immunotherapy, and CAR-T cell therapy. In addition to this, stem cell transplant is also used early in therapy for patients with high-risk subtypes of Acute Lymphoblastic Leukemia.
CAR-T Cell Therapy for Acute Lymphoblastic Leukemia Epidemiology
As the market is derived using a patient-based model, the CAR-T Cell Therapy for Acute Lymphoblastic Leukemia epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by total incident cases of Acute Lymphoblastic Leukemia, gender-specific cases of Acute Lymphoblastic Leukemia, age-specific cases of Acute Lymphoblastic Leukemia, subtype-specific cases of Acute Lymphoblastic Leukemia, genetic-mutation specific cases of Acute Lymphoblastic Leukemia, and total treated cases of Acute Lymphoblastic Leukemia in the 7MM covering the United States, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan from 2020 to 2034.
- Among the 7MM, the US accounted for the highest Acute Lymphoblastic Leukemia incidence.
- The total incident cases of Acute Lymphoblastic Leukemia in the US comprised approximately 6,800 cases in 2023 and are projected to increase during the forecast period.
- Amongst EU4 and the UK, Germany accounted for the highest number of incident cases of Acute Lymphoblastic Leukemia, while Spain accounted for the lowest number of cases.
- Among the gender-specific cases, males accounted for nearly 3,800 cases, while females accounted for 3,000 in the US in 2023.
- Among the type-specific cases of Acute Lymphoblastic Leukemia, the incident cases of B-Acute Lymphoblastic Leukemia accounted for nearly 85%, while that of T-Acute Lymphoblastic Leukemia accounted for nearly 15% in the US.
Unlock comprehensive insights! Click Here to Purchase the Full Epidemiology Report @ CAR T-Cell Therapy for Acute Lymphoblastic Leukemia Incidence
CAR-T Cell Therapy for Acute Lymphoblastic Leukemia Market Recent Developments and Breakthroughs
- In February 2025, MB-105, a first-in-class CD5-targeted CAR T-cell therapy, received orphan drug designation for treating relapsed/refractory (R/R) CD5-positive T-cell lymphoma. In a phase 1 study (NCT03081910), MB-105 is being evaluated in patients with T-cell acute lymphoblastic leukemia and R/R T-cell lymphoma, showing promising response rates in T-cell lymphoma cases.
- In January 2025, Autolus Therapeutics announced the FDA approval of its first product, AUCATZYL, a potentially best-in-class CD19 CAR T cell therapy for treating relapsed or refractory B-cell precursor acute lymphoblastic leukemia. The approval, supported by the FELIX trial, underscores the therapy’s novel mechanism of action and favorable safety profile, positioning Autolus for growth and market expansion in hemato-oncology.
- In November 2024, Autolus Therapeutics (Nasdaq: AUTL) announced that the FDA has approved AUCATZYL® (obecabtagene autoleucel) for treating adult patients with relapsed or refractory B-cell precursor acute lymphoblastic leukemia (r/r B-ALL).
- In September 2024, Vironexis Biotherapeutics announced the clearance of its Investigational New Drug (IND) application by the FDA for VNX-101, its first AAV-delivered gene therapy product. VNX-101 is designed for the treatment of CD19+ acute lymphoblastic leukemia and has received Fast Track Designation and Rare Pediatric Disease Designation. The company plans to initiate a Phase 1/2 trial for VNX-101 in Q4 2024, marking the first clinical trial of an AAV-delivered cancer immunotherapy.
CAR-T Cell Therapy for Acute Lymphoblastic Leukemia Drugs Chapters
The drug chapter segment of the CAR-T Cell Therapy for Acute Lymphoblastic Leukemia treatment market report encloses a detailed analysis of marketed and late-stage (Phase III and Phase II) CAR-Ts. It also helps understand the CAR-T Cell Therapy for Acute Lymphoblastic Leukemia pivotal clinical trials details, recent and expected market approvals, patent details, advantages and disadvantages of each included drug, the latest CAR T-Cell Therapy for Acute Lymphoblastic Leukemia news, and recent deals and collaborations.
Marketed CAR-T Cell Therapy for Acute Lymphoblastic Leukemia Drugs
-
TECARTUS (brexucabtagene autoleucel): Gilead Sciences
TECARTUS is a CD19-directed genetically modified autologous T cell immunotherapy, which binds to CD19-expressing cancer cells and normal B cells. Studies demonstrated that following anti-CD19 CAR-T cell engagement with CD19-expressing target cells, the CD28 and CD3-zeta costimulatory domains activate downstream signaling cascades that lead to T cell activation, proliferation, acquisition of effector functions, and secretion of inflammatory cytokines and chemokines. This sequence of events leads to the killing of CD19-expressing cells. In October 2021, the US FDA approved TECARTUS for adult patients with R/R B-Acute Lymphoblastic Leukemia. It was approved by the EC in September 2022.
-
KYMRIAH (tisagenlecleucel): Novartis
KYMRIAH is a CD19-directed genetically modified autologous T cell immunotherapy that involves reprogramming a patient’s T cells with a transgene encoding a chimeric antigen receptor (CAR) to identify and eliminate CD19-expressing malignant and normal cells. The CAR is comprised of a murine single-chain antibody fragment that recognizes CD19 and is fused to intracellular signaling domains. Upon binding to CD19-expressing cells, the CAR transmits a signal to promote T cell expansion, activation, target cell elimination, and persistence of the KYMRIAH cells. In August 2017, the US FDA approved KYMRIAH for the treatment of patients up to age 25 years with R/R B-Acute Lymphoblastic Leukemia. It was approved by the EC in August 2018, and by the MHLW in March 2019.
CAR-T Cell Therapy for Acute Lymphoblastic Leukemia Emerging Drugs
-
Obecabtagene autoleucel (obe-cel): Autolus Therapeutics
Obe-cel is an autologous CD19 CAR-T cell therapy with a unique CD19 CAR. The CAR is designed to have a fast-off kinetic, which mimics physiological T-cell receptor interactions. In March 2022, obe-cel was granted Orphan Medical Product Designation by the EMA for the treatment of Acute Lymphoblastic Leukemia, having previously received ODD by the US FDA for B-Acute Lymphoblastic Leukemia. In April 2022, the US FDA granted Regenerative Medicine Advanced Therapy (RMAT) designation to obe-cel for the treatment of adult B-Acute Lymphoblastic Leukemia. Obe-cel also received PRIME designation from the EMA and Innovative Licensing and Access Pathway (ILAP) by the Medicines and Healthcare Products Regulatory Agency (MHRA), UK. Recently, in January 2024, the US FDA has accepted its BLA for obecabtagene autoleucel for patients with r/r Acute Lymphoblastic Leukemia. Under the Prescription Drug User Fee Act (PDUFA), the FDA has set a target action date of November 16, 2024. The BLA submission is based on data from the Pivotal Phase II FELIX study of obe-cel in adult r/r B-Acute Lymphoblastic Leukemia. The data were presented at the 2023 American Society of Clinical Oncology (ASCO) Annual Meeting in June 2023, with updated data presented at the Annual Meeting of the American Society for Hematology Meeting (ASH) in December 2023.
-
UCART22: Cellectis
UCART22 is an allogeneic-engineered T-cell product candidate designed for the treatment of CD22-expressing hematologic malignancies and is currently being developed for the treatment of B-Acute Lymphoblastic Leukemia. Currently, the company is evaluating the drug in Phase I/II B Acute Lymphoblastic Leukemia I-01 Study in patients with R/R Acute Lymphoblastic Leukemia. As of the December 2021 report, the company is enrolling patients in the third dose level of the B Acute Lymphoblastic Leukemia I-01 Study with a fludarabine cyclophosphamide alemtuzumab lymphodepletion regimen.
|
Comparison of Emerging CAR-T Cell Therapy for Acute Lymphoblastic Leukemia | |||||||
|
Product |
Company |
Phase |
Indication |
Designation |
MoA |
RoA |
Molecule Type |
|
UCART22 |
Cellectis |
I/II |
R/R CD22+ B-Acute Lymphoblastic Leukemia |
- |
T lymphocyte replacements |
IV |
Allogeneic CAR-T cell therapy |
|
WU-CART-007 |
Wugen |
I/II |
R/R T-Acute Lymphoblastic Leukemia |
FTD, RPDD, ODD |
T lymphocyte replacements |
IV infusion |
Allogeneic CAR-T cell therapy |
|
Obecabtagene autoleucel |
Autolus Therapeutics |
I/II |
R/R B-Acute Lymphoblastic Leukemia |
RMAT, ODD, PRIME, PIM, ILAP |
Targeting CD19 cells |
IV infusion |
Autologous CAR-T cell therapy |
|
TBI-1501 |
Takara Bio |
I/II |
B-Acute Lymphoblastic Leukemia |
- |
Targeting and binding to CD19 expressing neoplastic B cells |
IV infusion |
Autologous CD19-CAR-T cell therapy |
|
PBCAR0191 |
Imugene/Precision Biosciences |
I/II |
R/R B-Acute Lymphoblastic Leukemia |
FTD |
T lymphocyte replacements |
IV infusion |
Allogeneic CAR-T cell therapy |
|
BREYANZI (lisocabtagene maraleucel) |
Bristol Myers Squibb |
I/II |
R/R B-Acute Lymphoblastic Leukemia |
- |
Targeting CD19 cells |
IV infusion |
Autologous CAR-T cell therapy |
|
PMB-CT01 |
PeproMene Bio |
I |
R/R B-Acute Lymphoblastic Leukemia |
- |
Targeting BAFF-R signaling |
IV infusion |
Autologous CAR-T cell therapy |
|
AUTO1/22 |
Autolus Therapeutics |
I |
HR-relapsed Acute Lymphoblastic Leukemia |
- |
Dual targeting CD19 and CD22 cells |
IV infusion |
Autologous CAR-T cell therapy |
|
UCART19 |
Servier/Allogene |
I |
R/R B-Acute Lymphoblastic Leukemia |
- |
Allogeneic anti-CD19 CAR T |
IV infusion |
Allogenic CAR-T cell therapy |
|
FT819 |
Fate Therapeutics |
I |
B-Acute Lymphoblastic Leukemia |
- |
Targeting CD19 cells |
IV infusion |
Allogenic CAR-T cell therapy |
CAR-T Cell Therapy for Acute Lymphoblastic Leukemia Market Outlook
Traditional cytotoxic chemotherapy-containing regimens have been the backbone of treatment for adults with Acute Lymphoblastic Leukemia for decades. Common complications of traditional chemotherapy can be fatal and include infection, bleeding, thrombosis, neuropathy, osteonecrosis, and the development of secondary cancers, including AML and MDS.
Since the first successful report of CAR-T cell therapy for pediatric Acute Lymphoblastic Leukemia more than a decade ago, the field has exploded with new constructs and targets, insights into CAR T-cell persistence, and novel antigen escape mechanisms; first came KYMRIAH (tisagenlecleucel, or tisa-cel, Novartis) for pediatric and young adult patients who had received at least two prior lines of therapy, followed by TECARTUS (brexucabtagene autoleucel, or brexu-cel, Kite) for adults following first relapse. It is anticipated that CAR-T cells will bring out the next big leap forward in leukemia immunotherapy.
Obecabtagene autoleucel (obe-cel; AUTO1), a novel, second-generation CAR product, is currently the most advanced candidate in the CAR-T in the Acute Lymphoblastic Leukemia emerging pipeline. CD19 negative relapse is a major cause of treatment failure after CD19 CAR T-cell therapy for pediatric B-Acute Lymphoblastic Leukemia. To address this, Autolus has designed AUTO1/22 to target both CD19 and CD22 using the fast-off rate CD19 CAR from obecabtagene autoleucel (obe-cel) combined with a novel CD22 CAR capable of effective signaling in response to low antigen density.
Several advancing allogeneic CAR-T cell therapies in Acute Lymphoblastic Leukemia Companies such as Precision Biosciences (PBCAR0191), Cellectis (UCART22), Wugen (WU-CART-007), and Cellectis/Servier/Allogene (UCART19), and others.
- The total CAR T-Cell Therapy for Acute Lymphoblastic Leukemia Market Size in the 7MM was ~USD 200 million in 2023 and is projected to increase during the forecast period (2024–2034).
- Amongst EU4 and the UK, Germany accounted for the largest CAR T-Cell Therapy for Acute Lymphoblastic Leukemia market size in 2023, while Spain occupied the bottom of the ladder.
- Among the therapies, TECARTUS is expected to generate the highest revenue in the 7MM by 2034.
CAR-T Cell Therapy for Acute Lymphoblastic Leukemia Drugs Uptake
This section focuses on the rate of uptake of the potential CAR-Ts drugs expected to be launched in the market during the study period 2020-2034. The analysis covers market uptake by CAR-Ts; patient uptake by CAR-Ts; and sales of each CAR-T Cell Therapy for Acute Lymphoblastic Leukemia.
CAR-T Cell Therapy for Acute Lymphoblastic Leukemia Pipeline Development Activities
The CAR T-Cell Therapy for Acute Lymphoblastic Leukemia treatment market report provides insights into CAR-T Cell Therapy for Acute Lymphoblastic Leukemia clinicla trials within Phase III and Phase II. It also analyzes key CAR T-Cell Therapy for Acute Lymphoblastic Leukemia Companies involved in developing targeted therapeutics.
Pipeline Development Activities
The CAR T-Cell Therapy for Acute Lymphoblastic Leukemia treatment market report covers information on collaborations, acquisitions and mergers, licensing, and patent details for CAR-T Cell Therapy for Acute Lymphoblastic Leukemia emerging therapies.
Latest KOL Views on CAR-T Cell Therapy for Acute Lymphoblastic Leukemia
To keep up with current CAR T-Cell Therapy for Acute Lymphoblastic Leukemia market trends, we take KOLs and SMEs’ opinions working in the domain through primary research to fill the data gaps and validate our secondary research. Industry experts contacted for insights on CAR-T Cell Therapy for Acute Lymphoblastic Leukemia evolving CAR T-Cell Therapy for Acute Lymphoblastic Leukemia treatment market landscape, patient reliance on conventional therapies, patient’s therapy switching acceptability, and drug uptake, along with challenges related to accessibility, including Medical/scientific writers; American Cancer Society; Hematologist and Professors; MD, FACS, Chair of the Department of Department of Hematology, University of Texas MD Anderson Cancer Center, and others.
Delveinsight’s analysts connected with 30+ KOLs to gather insights; however, interviews were conducted with 10+ KOLs in the 7MM. Their opinion helps understand and validate current and emerging therapy treatment patterns or CAR-T Cell Therapy for Acute Lymphoblastic Leukemia market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the CAR T-Cell Therapy for Acute Lymphoblastic Leukemia treatment market and the CAR T-Cell Therapy for Acute Lymphoblastic Leukemia unmet needs.
Qualitative Analysis
We perform Qualitative and CAR T-Cell Therapy for Acute Lymphoblastic Leukemia market Intelligence analysis using various approaches, such as SWOT analysis and Analyst views. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the Analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving treatment landscape.
The analyst analyzes multiple emerging CAR T-Cell Therapy for Acute Lymphoblastic Leukemia therapies based on relevant attributes such as safety, efficacy, target population, and order of entry. In efficacy, the trial’s primary and secondary outcome measures are evaluated. Further, the therapies’ safety is evaluated wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials.
CAR T-Cell Therapy for Acute Lymphoblastic Leukemia Treatment Market Access and Reimbursement
Reimbursement is a crucial factor affecting the drug’s market access. Often, the decision to reimburse comes down to the price of the drug relative to the benefit it produces in treated patients. To reduce the healthcare burden of these high-cost therapies, payers and other industry insiders are considering many payment models. Understanding insurance and out-of-pocket costs shouldn’t be overwhelming.
Patients whose healthcare professionals have prescribed TECARTUS therapy can work with Kite Konnect. This integrated technology platform provides information and assistance throughout the therapy process for Kite’s commercialized CAR-T therapies, including courier tracking for shipments and manufacturing status updates. Kite Konnect provides support for eligible patients receiving TECARTUS, and it includes information for the healthcare teams supporting their patients.
The report further provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of approved therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
CAR-T Cell Therapy for Acute Lymphoblastic Leukemia Treatment Market Report Scope
- The CAR T-Cell Therapy for Acute Lymphoblastic Leukemia therapeutics market report covers a segment of key events, an executive summary, and a descriptive overview, explaining its causes, signs, symptoms, pathogenesis, and currently used CAR-T therapies.
- Comprehensive insight into the epidemiology segments and forecasts, disease progression, and treatment guidelines has been provided.
- Additionally, an all-inclusive account of the emerging therapies and the elaborative profiles of late-stage and prominent therapies will impact the current CAR T-Cell Therapy for Acute Lymphoblastic Leukemia treatment market landscape.
- A detailed review of the CAR-T in the Acute Lymphoblastic Leukemia market, historical and forecasted CAR T-Cell Therapy for Acute Lymphoblastic Leukemia market size, CAR T-Cell Therapy for Acute Lymphoblastic Leukemia market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM therapy outreach.
- The CAR T-Cell Therapy for Acute Lymphoblastic Leukemia treatment market report provides an edge while developing business strategies, by understanding trends, through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help shape and drive the 7MM of CAR-T Cell Therapy for Acute Lymphoblastic Leukemia.
CAR-T Cell Therapy for Acute Lymphoblastic Leukemia Treatment Market Report Insights
- Patient-based CAR T-Cell Therapy for Acute Lymphoblastic Leukemia Market Forecasting
- CAR-T Cell Therapy for Acute Lymphoblastic Leukemia Therapeutic Approaches
- CAR-T Cell Therapy for Acute Lymphoblastic Leukemia Pipeline Analysis
- CAR-T Cell Therapy for Acute Lymphoblastic Leukemia Market Size
- CAR T-Cell Therapy for Acute Lymphoblastic Leukemia Market Trends
- Existing and Future CAR T-Cell Therapy for Acute Lymphoblastic Leukemia Drugs Market Opportunity
CAR-T Cell Therapy for Acute Lymphoblastic Leukemia Treatment Market Report Key Strengths
- 10 Years CAR T-Cell Therapy for Acute Lymphoblastic Leukemia Market Forecast
- The 7MM Coverage
- CAR-T Cell Therapy for Acute Lymphoblastic Leukemia Epidemiology Segmentation
- Key Cross Competition
- Conjoint Analysis
- CAR-T Cell Therapy for Acute Lymphoblastic Leukemia Drugs Uptake
- Key CAR-T Cell Therapy for Acute Lymphoblastic Leukemia Market Forecast Assumptions
CAR-T Cell Therapy for Acute Lymphoblastic Leukemia Market Report Assessment
- Current CAR-T Cell Therapy for Acute Lymphoblastic Leukemia Treatment Market Practices
- CAR-T Cell Therapy for Acute Lymphoblastic Leukemia Unmet Needs
- CAR-T Cell Therapy for Acute Lymphoblastic Leukemia Pipeline Product Profiles
- CAR-T Cell Therapy for Acute Lymphoblastic Leukemia Drugs Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
- CAR-T Cell Therapy for Acute Lymphoblastic Leukemia Market Drivers
- CAR-T Cell Therapy for Acute Lymphoblastic Leukemia Market Barriers
FAQs
- What was the CAR-T Cell Therapy for Acute Lymphoblastic Leukemia Drugs Market share (%) distribution in 2020, and what would it look like in 2034?
- At what CAGR is the CAR-T Cell Therapy for Acute Lymphoblastic Leukemia Drugs Market expected to grow in the 7MM during the study period (2020–2034)?
- Among the emerging CAR-Ts, which potential CAR-T is expected to disrupt the Acute Lymphoblastic Leukemia markets?
- What will be the impact on the sales of TECARTUS and KYMRIAH after the entry of its generics into the market?
- What are the disease risks, burdens, and CAR T-Cell Therapy for Acute Lymphoblastic Leukemia unmet needs? What will be the growth opportunities across the 7MM concerning the patient population?
- Which emerging CAR-T is going to garner the maximum CAR T-Cell Therapy for Acute Lymphoblastic Leukemia market share in the 7MM in 2034?
- What are the various recent and upcoming events expected to improve the uptake of CAR-T Cell Therapy for Acute Lymphoblastic Leukemia?
- How much market share will be captured by CAR-Ts by 2034?
- What are the current guidelines for treating Acute Lymphoblastic Leukemia using CAR-Ts in the US, Europe, and Japan?
- What are the recent novel therapies, targets, mechanisms of action, and technologies being developed to overcome the limitations of existing therapies?
- How is the access to CAR-Ts among different countries?
Reasons to Buy the CAR-T Cell Therapy for Acute Lymphoblastic Leukemia Market Report
- The CAR T-Cell Therapy for Acute Lymphoblastic Leukemia drugs market report will help develop business strategies by understanding the latest trends and changing treatment dynamics driving the CAR-T Cell Therapy for Acute Lymphoblastic Leukemia.
- Insights on patient burden/disease, CAR T-Cell Therapy for Acute Lymphoblastic Leukemia Incidence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years.
- Understand the existing CAR T-Cell Therapy for Acute Lymphoblastic Leukemia Drugs Market opportunities in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
- Identifying strong upcoming players in the CAR T-Cell Therapy for Acute Lymphoblastic Leukemia Drugs Market will help devise strategies to help get ahead of competitors.
- To understand the future market competition in the CAR-T Cell Therapy for Acute Lymphoblastic Leukemia market.
- Highlights of access and reimbursement policies of current therapies, barriers to accessibility of expensive off-label therapies, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing CAR T-Cell Therapy for Acute Lymphoblastic Leukemia Drugs Market so that the upcoming players can strengthen their development and launch strategy.
Stay Updated with us for Recent Articles @ Latest DelveInsight Blogs
-market.png&w=256&q=75)