CAR T-Cell Therapy for Multiple Myeloma Market
- The standard treatment options for multiple myeloma typically involve a combination of pain relievers, antibiotics, chemotherapy, immunotherapy, radiation therapy, targeted therapy, and bone marrow transplantation.
- CAR-T cell therapy is a personalized cancer treatment that modifies a patient’s own immune T cells in the lab to recognize and attack cancer cells more effectively. These engineered cells are infused back into the patient to target and kill cancer cells, especially in certain blood cancers like multiple myeloma.
- Further, CAR-T cell therapy offers a novel treatment option for multiple myeloma. Currently, there are two FDA-approved CAR-T therapies for the indication that includes ABECMA (idecabtagene vicleuceland) and CARVYKTI (ciltacabtagene autoleucel).
- Two CAR-T therapies are approved for the treatment of Multiple myeloma. Although CAR-T treatments have demonstrated significant effectiveness, they also come with safety issues such as CRS. Cost, convenience, and manufacturing turnaround time initially could prevent CAR-T from being widely adopted, but over time, companies may reduce side effects and speed up manufacturing time.
- The heavy-pretreatment multiple myeloma space is more focused on CAR T-cell therapies. In the same segment Bispecific antibodies have also been approved. With this it is common to compare CAR T-cell therapies and the bispecific antibodies. Between CAR-Ts and bispecifics, everyone wants CAR-Ts; yet, CAR-Ts are not without difficulties. Given the high ORR seen among all CAR T-cell therapies and bispecific antibodies the research is continued to understand the sequential treatment for all these agents.
- CARVYKTI became the first and only BCMA-targeted treatment approved by the US FDA for patients with relapsed or refractory multiple myeloma who have received at least one prior line of therapy. As of right now, CARVYKTI is the most lucrative approved CAR-T treatment for multiple myeloma. Sales in the first seven quarters of CARVYKTI surpassed the historical CAR-T launches.
- The emerging pipeline for CAR-T cell therapy for multiple myeloma includes Anitocabtagene autoleucel (Arcellx/Kite), Zevor-cel (zevorcabtagene autoleucel) (CARsgen Therapeutics), and Arlocabtagene autoleucel (Juno Therapeutics/ Bristol-Myers Squibb).
- In June 2025, Arcellx announced new positive results from its pivotal Phase II iMMagine-1 trial evaluating anitocabtagene autoleucel (anito-cel) in patients with relapsed or refractory multiple myeloma (RRMM). The findings were presented in an oral session at the EHA2025 Congress in Milan on June 14, 2025.
- In June 2024, CARsgen Therapeutics presented the updated results from LUMMICAR STUDY 1 of zevorcabtagene autoleucel at 29th European Hematology Association (EHA) Annual Congress.
Request for Unlocking the Sample Page of the "CAR-T Cell Therapy for Multiple Myeloma Treatment Market"
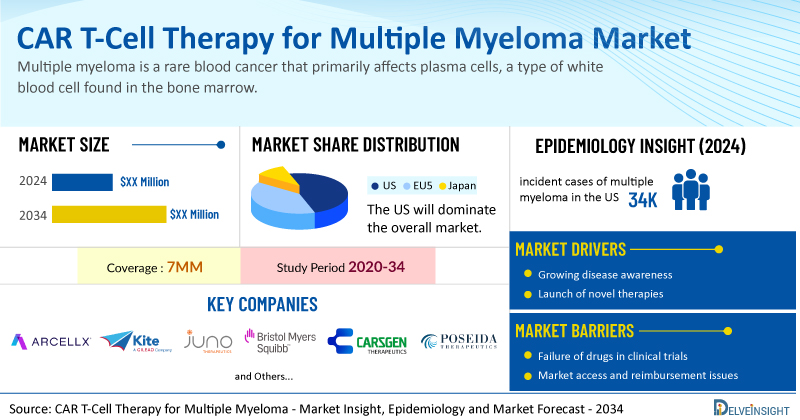
DelveInsight's “Chimeric Antigen Receptor (CAR)-T Cell Therapy for Multiple Myeloma Market Insight, Epidemiology and Market Forecast 2034” report delivers an in-depth analysis of CAR-T cell therapy for multiple myeloma market, and clinical development in CAR-T cell therapy for multiple myeloma. In addition to this, the report provides historical and forecasted epidemiology and market data as well as a detailed analysis of the CAR-T cell therapy for multiple myeloma market trends in the United States, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
CAR-T cell therapy for multiple myeloma market report provides real-world prescription pattern analysis, emerging drugs assessment, market share, and uptake/adoption pattern of individual therapies, as well as historical and forecasted CAR-T cell therapy for multiple myeloma market size from 2020 to 2034 in 7MM. The report also covers current CAR-T cell therapy for multiple myeloma treatment practices/algorithms and unmet medical needs to curate the best opportunities and assess the market’s underlying potential.
|
Study Period |
2020–2034 |
|
Forecast Period |
2025–2034 |
|
Geographies Covered |
US, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan |
|
CAR-T Cell Therapy for Multiple Myeloma Epidemiology
|
Segmented by:
|
|
CAR-T Cell Therapy for Multiple Myeloma Companies |
|
|
CAR-T Cell Therapy for Multiple Myeloma Therapies |
|
|
CAR-T Cell Therapy for Multiple Myeloma Market |
Segmented by:
|
|
Analysis |
|
Key Factors Driving the Growth of the CAR T-Cell Therapy for Multiple Myeloma Market
Rising Multiple Myeloma Incidence
The increasing number of multiple myeloma cases worldwide is a significant driver for CAR T-cell therapy adoption. In the US alone, over 32,000 new cases are diagnosed annually, highlighting the growing need for effective treatments. According to DelveInsight analysis, among the 7MM, the highest number of incident cases was reported in the US in 2024, at nearly 34,000, which are further expected to increase by 2034.
Efficacy of BCMA-Targeted Therapies
BCMA-targeted CAR T-cell therapies, such as Bristol Myers Squibb/Bluebird Bio’s ABECMA and Johnson & Johnson’s CARVYKTI, have demonstrated high response rates in treating patients with relapsed or refractory multiple myeloma. These therapies offer new hope for patients who have exhausted other treatment options.
Rising CAR T-Cell Therapy for Multiple Myeloma Clinical Trial Activity
Some of the prominent players in this field include Arcellx and Kite (a Gilead company) (anitocabtagene autoleucel, anito-cel), CARsgen Therapeutics (Zevor-cel, zevorcabtagene autoleucel), Kelonia Therapeutics (KLN-1010), Poseida Therapeutics/Roche (P-BCMA-ALLO1), Juno Therapeutics (Bristol Myers Squibb) (arlocabtagene autoleucel), Gracell Biopharmaceuticals (GC012F), Caribou Biosciences (CB-011), and others.
Chimeric Antigen Receptor (CAR)-T Cell Therapy for Multiple Myeloma Understanding and Treatment Algorithm
Multiple myeloma is a rare type of blood cancer that affects plasma cells (a type of white blood cells) in the bone marrow. Normal plasma cells produce antibodies or immunoglobulins which fight against infection. In multiple myeloma, these cells proliferate uncontrollably, crowding out normal cells and producing large amounts of abnormal antibody proteins known as monoclonal protein (M protein) or paraprotein. This can result in complications such as pain in bone, thrombocytopenia, leukopenia, destruction of bone, anemia, renal impairment and hypercalcaemia. The exact cause of multiple myeloma is unknown; however, several risk factors associated with its development including age, race, sex, family history, exposure to radiation and certain chemicals.
CAR-T Cell Therapy is a next generation immunotherapy that uses a patient's own immune cells to fight cancer. It involves extracting T-cells from the patient, modifying them to express a CAR that recognizes cancer-specific antigens, multiplying these CAR T-cells, and then infusing them back into the patient. This allows the modified T-cells to specifically target and kill cancer cells, offering a potentially curative treatment option for certain types of blood cancers.
Further details related to treatment will be provided in the report…
Chimeric Antigen Receptor (CAR)-T Cell Therapy for Multiple Myeloma Epidemiology
The CAR-T cell therapy for multiple myeloma epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented as total incident cases of multiple myeloma, total eligible cases of multiple myeloma for CAR-T cell therapies, and total treatable cases of multiple myeloma for CAR-T cell therapies in the 7MM covering the United States, EU4 (Germany, France, Italy, and Spain), and the United Kingdom, and Japan from 2020 to 2034.
- In the 7MM, the highest incident cases of multiple myeloma were seen in the United States, followed by EU4 and the UK.
- Among EU4, Germany accounted for the highest cases of incident multiple myeloma cases, whereas Spain accounted for the lowest cases in 2024.
- Multiple myeloma is more common in males as compared to females. More than 50% of males in the United States are diagnosed with multiple myeloma.
- Data suggests that roughly half of newly diagnosed multiple myeloma patients are ineligible for transplant, and around a third of eligible patients do not receive the transplant.
Chimeric Antigen Receptor (CAR)-T Cell Therapy for Multiple Myeloma Drug Analysis
The drug chapter segment of CAR-T cell therapy for multiple myeloma report encloses a detailed analysis of CAR-T Cell therapy for multiple myeloma -marketed drugs and emerging pipeline drugs. It also deep dives into CAR-T cell therapy for multiple myeloma’s pivotal clinical trial details, recent and expected market approvals, patent details, the latest news, and recent deals and collaborations.
Chimeric Antigen Receptor (CAR)-T Cell Therapy for Multiple Myeloma Marketed Drugs
ABECMA (idecabtagene vicleucel): Bristol Myers Squibb/Bluebird Bio
ABECMA is a CAR-T cell therapy that targets B-cell maturation antigen (BCMA), which is expressed on the surface of normal and malignant plasma cells. It is indicated for the treatment of adult patients with relapsed or refractory multiple myeloma (RRMM). Antigen-specific activation of ABECMA results in CAR-T cell proliferation, cytokine secretion, and subsequent cytolytic killing of BCMA-expressing cells. It has been granted Orphan Drug and Breakthrough Therapy designations in the US and Europe. In April 2024, the FDA approved ABECMA for the treatment of adult patients with relapsed or refractory multiple myeloma after two or more prior lines of therapy including an immunomodulatory agent (IMiD), a proteasome inhibitor (PI), and an anti-CD38 monoclonal antibody, based on results from the KarMMa-3 trial.
CARVYKTI (ciltacabtagene autoleucel): Johnson & Johnson
CARVYKTI is a BCMA-directed genetically modified autologous T cell immunotherapy indicated for the treatment of adult patients with RRMM. It involves reprogramming a patient’s own T cells with a transgene encoding a CAR that identifies and eliminates cells that express BCMA. CARVYKTI received Orphan Drug designation from the US FDA and the European Commission, Breakthrough Therapy designation from FDA. In April 2024, Johnson & Johnson announced that the US FDA approved CARVYKTI for the treatment of adult patients with RRMM who have received at least one prior line of therapy, including a proteasome inhibitor and an immunomodulatory agent, and are refractory to lenalidomide.
| Comparison of Chimeric Antigen Receptor (CAR)-T Cell Therapy for Multiple Myeloma Marketed Drugs | ||||||
|
Drug Name |
Company |
Indication |
RoA |
MoA |
Molecule Type | |
|
ABECMA |
Bristol Myers Squibb/Bluebird Bio |
RRMM |
IV infusion |
Targets BCMA |
CAR T-cell therapy | |
|
CARVYKTI |
Johnson & Johnson |
RRMM |
IV infusion |
Targets BCMA |
CAR T-cell therapy | |
Chimeric Antigen Receptor (CAR)-T Cell Therapy for Multiple Myeloma Emerging Drugs
Arlocabtagene autoleucel: Juno Therapeutics/Bristol-Myers Squibb
Arlocabtagene autoleucel is an investigational GPRC5D-targeted CAR T-cell therapy for the treatment of patients with relapsed or refractory multiple myeloma. As a GPRC5D-targeting CAR T-cell therapy, arlocabtagene autoleucel represents a novel immunotherapy approach focused on patients who have exhausted multiple prior lines of treatment.
It is currently being evaluated in a Phase III (QUINTESSENTIAL-2) clinical trial in adult patients with relapsed or refractory multiple myeloma.
In June 2025, Bristol-Myers Squibb presented the data across its oncology portfolio and pipeline including Phase III trial of Arlocabtagene autoleucel in 2025 American Society of Clinical Oncology (ASCO).
In December 2024, Bristol Myers Squibb presented significant advancements in its expanded pipeline, highlighting data across multiple therapeutic modalities. Notably, the company shared the first survival results for arlocabtagene autoleucel, in patients with relapsed/refractory multiple myeloma. These results, unveiled at the American Society of Hematology (ASH) 2024 Annual Meeting, demonstrated promising long-term survival outcomes, underscoring the therapy's potential impact.
Anitocabtagene autoleucel (anito-cel): Arcellx/Kite
Anitocabtagene autoleucel is a BCMA CAR T that utilizes a novel binder (or CAR) known as the D-Domain. It is being investigated for the treatment in patients with relapsed or refractory multiple myeloma who have received 1-3 prior lines of therapy and have been previously exposed to both an immunomodulatory drug (IMiD) and an anti-CD38 antibody. It has been granted Fast Track, Orphan Drug, and Regenerative Medicine Advanced Therapy designations by the US FDA.
Currently, anitocabtagene autoleucel is being evaluated in Phase III (iMMagine-3) clinical trial.
- In May 2025, Arcellx announced new positive results from its pivotal Phase II iMMagine-1 trial evaluating anitocabtagene autoleucel (anito-cel) in patients with relapsed or refractory multiple myeloma (RRMM). The updated data are scheduled to be presented in an oral session at the EHA2025 Congress in Milan on June 14, 2025.
|
Comparison of Emerging Drugs Under Development | ||||||
|
Drug Name |
Company |
Highest Phase |
Indication |
RoA |
MoA |
Molecule Type |
|
Anitocabtagene autoleucel |
Arcellx/Kite |
III |
RRMM |
IV infusion |
Targets BCMA |
Autologous CAR T cell therapy |
|
Arlocabtagene autoleucel |
Juno Therapeutics/ Bristol-Myers Squibb |
III |
RRMM |
IV infusion |
Targets GPRC5D |
Autologous CAR T cell therapy |
|
Zevor-cel (zevorcabtagene autoleucel) |
CARsgen Therapeutics |
I/II |
RRMM |
IV infusion |
Targets BCMA |
Autologous CAR T cell therapy |
|
P-BCMA-ALLO1 |
Poseida Therapeutics |
I |
RRMM |
IV infusion |
Targets BCMA |
Allogeneic CAR T cell therapy |
|
CB-011 |
Caribou Biosciences |
I |
RRMM |
IV infusion |
Caribou Biosciences |
Allogeneic CART-cell |
Note: Detailed list will be provided in the final report...
Chimeric Antigen Receptor (CAR)-T Cell Therapy for Multiple Myeloma Drug Class Analysis
BCMA-directed CAR T cell Therapy
BCMA-directed CAR T cell therapy is an innovative immunotherapy for multiple myeloma that involves modifying a patient’s own T cells to target B-cell maturation antigen (BCMA), a protein abundantly expressed on malignant plasma cells. This approach involves engineering autologous T cells with chimeric antigen receptors (CARs) specific for BCMA to recognize and eliminate myeloma cells. It has shown significant effectiveness in treating relapsed or refractory multiple myeloma. Further, the therapy leverages BCMA’s selective expression and essential role in the growth and survival of myeloma cells, making it an ideal therapeutic target. However, challenges such as antigenic escape, cytokine release syndrome, and logistical complexities remain under ongoing research and clinical refinement.
Chimeric Antigen Receptor (CAR)-T Cell Therapy for Multiple Myeloma Market Outlook
CAR-T cell therapy is emerging as a promising treatment for multiple myeloma, especially in patients with relapsed or refractory disease. These therapies are directed against B-cell maturation antigen (BCMA), a protein expressed on the surface of multiple myeloma cells. By specifically targeting BCMA, they have demonstrated significant efficacy, achieving high response rates in patients with relapsed or refractory multiple myeloma who have previously undergone and exhausted other treatment modalities. Currently, there are two FDA-approved CAR T-cell therapies ABECMA (idecabtagene vicleuceland) and CARVYKTI (ciltacabtagene autoleucel) for treatment of patients with relapsed or refractory multiple myeloma.
Further, CAR-T cell therapy is a novel innovative therapy; thus, there are only few drugs which are currently under clinical development for multiple myeloma, including Anitocabtagene autoleucel (Arcellx/Kite), Zevor-cel (zevorcabtagene autoleucel) (CARsgen Therapeutics), and Arlocabtagene autoleucel (Juno Therapeutics/ Bristol-Myers Squibb).
Arlocabtagene autoleucel is an investigational CAR-T cell therapy directed against GPRC5D, which differs from the currently approved BCMA-targeted CAR-T cell therapies. Its development may expand treatment options for patients who have developed resistance or intolerance to BCMA-directed CAR T therapies. Ongoing clinical development and anticipated regulatory approvals of GPRC5D-targeted CAR-T therapies are expected to significantly influence and accelerate the market growth of CAR-T cell therapy for multiple myeloma in the near future.
Chimeric Antigen Receptor (CAR)-T Cell Therapy for Multiple Myeloma Drug Uptake
This section focuses on the uptake rate of potential drugs expected to be launched in the market during 2025–2034. The landscape of multiple myeloma treatment has experienced a profound transformation with the uptake of novel medicines. These innovative therapies are redefining standards of care.
Further detailed analysis of emerging therapies' drug uptake in the report.
Chimeric Antigen Receptor (CAR)-T Cell Therapy for Multiple Myeloma Pipeline Development Activities
The report provides insights into different therapeutic candidates in the marketed and emerging stages. It also analyses key players involved in developing targeted therapeutics.
Pipeline Development Activities
The report covers information on collaborations, acquisitions and mergers, licensing, and patent details CAR-T cell therapy for multiple myeloma.
Latest KOL Views
To keep up with the real-world scenario in current and emerging market trends, we take opinions from Key Industry leaders working in the domain through primary research to fill the data gaps and validate our secondary research. Industry Experts were contacted for insights on the evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake, along with challenges related to accessibility, including Medical/scientific writers, Professors, and others.
DelveInsight’s analysts connected with 15+ KOLs to gather insights; however, interviews were conducted with 10+ KOLs in the 7MM. Centers such as Stanford Hospital, Kyoto Prefectural University of Medicine, UT Southwestern Medical Center, etc., were contacted. Their opinion helps understand and validate current and emerging therapy treatment patterns or chimeric antigen receptor (CAR)-t cell therapy for multiple myeloma market trends.
Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT analysis and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of gaps in disease diagnosis, patient awareness, physician acceptability, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided.
Conjoint Analysis analyzes multiple approved and emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy.
In efficacy, the trial’s primary and secondary outcome measures are evaluated; for instance, in event-free survival, one of the most important primary outcome measures is event-free survival and overall survival.
Further, the therapies’ safety is evaluated wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Market Access and Reimbursement
Reimbursement may be referred to as the negotiation of a price between a manufacturer and a payer that allows the manufacturer access to the market. It is provided to reduce the high costs and make the essential drugs affordable. Health technology assessment (HTA) plays an important role in reimbursement decision-making and recommending the use of a drug. These recommendations vary widely throughout the seven major markets, even for the same drug. In the US healthcare system, both Public and Private health insurance coverage are included. Also, Medicare and Medicaid are the largest government-funded programs in the US. The major healthcare programs, including Medicare, Medicaid, Health Insurance Program (CHIP), and the state and federal health insurance marketplaces, are overseen by the Centers for Medicare & Medicaid Services (CMS). Other than these, Pharmacy Benefit Managers (PBMs) and third-party organizations that provide services and educational programs to aid patients are also present.
The report further provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of currently used therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Scope of the Chimeric Antigen Receptor (CAR)-T Cell Therapy for Multiple Myeloma Market Report
- The report covers a segment of key events, an executive summary, a descriptive overview of multiple myeloma, explaining its causes, signs and symptoms, pathogenesis, and currently available therapies.
- Comprehensive insight has been provided into the epidemiology segments and forecasts, the future growth potential of the diagnosis rate, and treatment guidelines.
- Additionally, an all-inclusive account of both the current and emerging therapies, along with the elaborative profiles of late-stage and prominent therapies, will have an impact on the current treatment landscape.
- A detailed review of the CAR-T cell therapy for multiple myeloma market, historical and forecasted market size, market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The report provides an edge while developing business strategies, by understanding trends, through SWOT and conjoint analysis and expert insights/KOL views, patient journey, and treatment preferences that help in shaping and driving the 7MM CAR-T cell therapy for multiple myeloma market.
Chimeric Antigen Receptor (CAR)-T Cell Therapy for Multiple Myeloma Report Insights
- Patient Population
- Therapeutic Approaches
- CAR-T Cell Therapy for Multiple Myeloma Pipeline Analysis
- CAR-T Cell Therapy for Multiple Myeloma Market Size and Trends
- Existing and Future Market Opportunity
Chimeric Antigen Receptor (CAR)-T Cell Therapy for Multiple Myeloma Report Key Strengths
- Ten-Year Forecast
- 7MM Coverage
- CAR-T Cell Therapy for Multiple Myeloma Epidemiology Segmentation
- Key Cross Competition
- Conjoint analysis
- Drugs Uptake and Key Market Forecast Assumptions
Chimeric Antigen Receptor (CAR)-T Cell Therapy for Multiple Myeloma Report Assessment
- Current Treatment Practices
- Unmet Needs
- Pipeline Product Profiles
- Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint)
FAQs
- What was the CAR-T cell therapy for multiple myeloma total market size, the market size by therapies, market share (%) distribution in 2020, and what would it look like in 2034? What are the contributing factors for this growth?
- At what CAGR, CAR-T cell therapy for multiple myeloma market expected to grow at the 7MM level during the study period (2020–2034)?
- What are the disease risks, burdens, and unmet needs of multiple myeloma?
- What will be the growth opportunities across the 7MM with respect to the patient population pertaining to multiple myeloma?
- What is the historical and forecasted multiple myeloma patient pool in the United States, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan?
- What are the current options for the treatment of multiple myeloma? What are the current treatment guidelines for the treatment of multiple myeloma in the US and Europe?
- Which CAR-T therapy is expected to be the leading contributor to the multiple myeloma market in the coming year?
- How many companies are developing CAR-T therapies for the treatment of multiple myeloma?
- Which key designations have been granted for the emerging therapies for multiple myeloma?
- What is the cost burden of approved therapies on the patient?
- Patient acceptability in terms of preferred treatment options as per real-world scenarios?
- What are the country-specific accessibility issues of expensive, recently approved therapies? Focus on reimbursement policies.
Reasons to Buy
- The report will help in developing business strategies by understanding the latest trends and changing treatment dynamics and driving factors for CAR-T cell therapy for the multiple myeloma market.
- Insights on patient share/disease burden, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years.
- Understand the existing market opportunities in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
- Identifying upcoming players in the market will help devise strategies to help get ahead of competitors.
- Highlights of access and reimbursement policies of approved therapies, barriers to accessibility of expensive off-label therapies, and patient assistance programs.
- To understand KOLs’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing market so that the upcoming players can strengthen their development and launch strategy.
Stay Updated with us for Recent Articles @ Latest DelveInsight Blogs