Chemotherapy Induced Peripheral Neuropathy Market Summary
- The Chemotherapy-Induced Peripheral Neuropathy Market Size in the 7MM was around USD 1,500 million in 2023. The Chemotherapy Induced Peripheral Neuropathy Therapeutics Market is anticipated to witness a substantial positive shift owing to better uptake of existing drugs and raised awareness.
- Among the 7MM, the United States accounted for the largest Chemotherapy Induced Peripheral Neuropathy Market Size (around USD 900 million) in 2023, in comparison to EU4 (Germany, Spain, Italy, France), the United Kingdom, and Japan.
Chemotherapy-Induced Peripheral Neuropathy Market and Epidemiology Analysis
- Chemotherapy-induced peripheral neuropathy (CIPN) is a frequent, dose-dependent complication of anticancer drugs, including platinum, taxanes, epothilones, vinca alkaloids, and newer agents, such as bortezomib. It not only leads to dose reduction or discontinuation of treatment but also decreases the quality of life of cancer survivors.
- Chemotherapy Induced Peripheral Neuropathy presents clinically as deficits in sensory, motor, and sometimes autonomic function. Sensory disturbances range from a mild tingling sensation to spontaneous burning pain and hypersensitivity to stimuli.
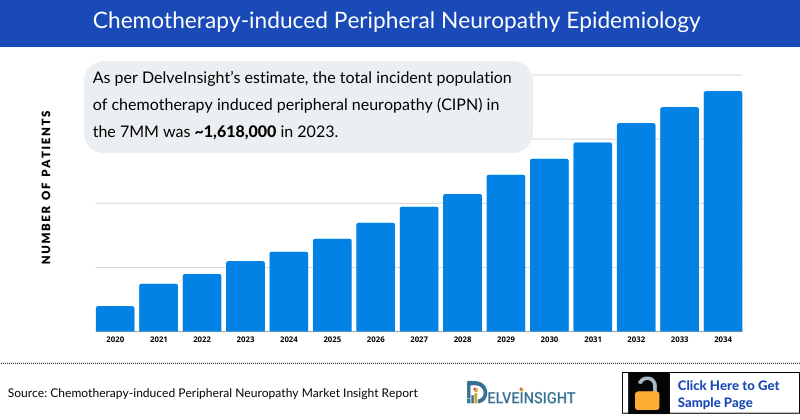
- As per DelveInsight’s estimate, the total Chemotherapy Induced Peripheral Neuropathy Incident Population in the 7MM was ~1,618,000 in 2023.
- The Chemotherapy Induced Peripheral Neuropathy incident cases based on chemotherapeutic agents in the US were maximum for platinum drugs, around 47%, followed by taxanes and vinca alkaloids, respectively.
- To this date, no therapeutic approach has adequately addressed the needs of patients, caregivers, oncologists, and pain specialists.
- The Chemotherapy Induced Peripheral Neuropathy Market is projected to grow by factors like an increase in the patient pool, projected entry of emerging therapy, i.e. Halneuron (Tetrodotoxin or TTX) and others, and their increasing usage in the treatment setting, in the 7MM.
- Peripheral neuropathy induced by cancer chemotherapy represents a large unmet need for patients due to the absence of treatment that can prevent or mitigate this common clinical problem. The Chemotherapy Induced Peripheral Neuropathy Market Size depends entirely on the type of nerve damage, symptoms, and location. Hence, a proper management strategy is necessary to enhance the patient outcomes and improve their quality of life (QoL).
Request for unlocking the sample page of the "Chemotherapy-Induced Peripheral Neuropathy Drugs Market"
Factors affecting Centronuclear Myopathy Market Growth
-
Rising Awareness and Diagnosis of Rare Neuromuscular Disorders
Increased understanding and recognition of rare genetic disorders, including CNM, are leading to earlier and more accurate diagnoses. This awareness drives demand for specialized therapies and diagnostic tools.
-
Advancements in Genetic Testing and Molecular Diagnostics
Innovations in next-generation sequencing (NGS) and genetic testing allow precise identification of CNM-related mutations, supporting early intervention and tailored treatment strategies.
-
Emergence of Targeted Therapies
Development of gene therapy, enzyme replacement therapy, and other novel treatments for CNM is expanding the therapeutic landscape, driving market growth.
-
Growing Investment in Rare Disease Research
Increasing funding and research initiatives for rare neuromuscular disorders encourage the development of new therapies and clinical trials, stimulating market expansion.
-
Increasing Supportive Care Measures
Advances in supportive care, including physiotherapy, respiratory support, and nutritional management, improve patient quality of life, enhancing the overall focus on CNM management.
-
Expansion of Healthcare Infrastructure in Emerging Markets
Improved access to genetic testing, neurology specialists, and treatment facilities in developing regions increases patient diagnosis and treatment adoption, boosting market growth.
-
Patient Advocacy and Awareness Programs
Efforts by rare disease advocacy groups to educate patients and caregivers about CNM are encouraging earlier diagnosis and access to available therapies, supporting market development
DelveInsight's “Chemotherapy-induced peripheral neuropathy Treatment Market Insights, Epidemiology and Market Forecast – 2034” report delivers an in-depth understanding of CIPN, historical and forecasted epidemiology as well as the CIPN market trends in the United States, EU4 (Germany, Spain, Italy, and France) and the United Kingdom, and Japan.
Chemotherapy Induced Peripheral Neuropathy Treatment Market report provides real-world prescription pattern analysis, emerging drugs, market share of individual therapies, and historical and forecasted 7MM Chemotherapy Induced Peripheral Neuropathy market size from 2020 to 2034. The report also covers current Chemotherapy Induced Peripheral Neuropathy treatment market practices/algorithms and unmet medical needs to curate the best opportunities and assess the market’s underlying potential.
|
Study Period |
2020 to 2034 |
|
Forecast Period |
2024-2034 |
|
Geographies Covered |
|
|
Chemotherapy Induced Peripheral Neuropathy Market |
|
|
Chemotherapy Induced Peripheral Neuropathy Market Size |
USD 1500 Million in 2023 |
|
Chemotherapy Induced Peripheral Neuropathy Companies |
|
Chemotherapy Induced Peripheral Neuropathy Treatment Market Disease Understanding
Chemotherapy-induced peripheral neuropathy (CIPN) is a common complication arising due to various anticancer medications, ranging from traditional ones like platinum and taxanes to newer agents like bortezomib. CIPN occurs in ~20% of patients given standard doses of chemotherapy and in almost 100% of patients treated with high doses. The complexity of neuromuscular assessment in CIPN poses challenges in evaluation and management. Various countries have developed a practical algorithm for diagnosis to aid oncology nurses in assessing and managing CIPN, employing techniques suitable for various clinical settings.
Some of the core diagnostic criteria for Chemotherapy-induced Peripheral Neuropathy are as follows-
- Onset of pain after exposure to a chemotherapeutic agent known to be neurotoxic.
- Painful symptoms are accompanied by nonpainful symptoms (e.g., ‘‘pins and needles’’ or numbness) in a similar distribution.
- Presence of painful symptoms in a symmetrical stocking and glove distribution beginning in lower extremities which may progress to the upper extremities, although finding in the feet and not in the hands is common.
- Magnitude of the sensory abnormalities is disproportionately greater than the magnitude of any motor abnormalities in the affected region (except in the case of neuropathy after vinca alkaloids).
Chemotherapy-Induced Peripheral Neuropathy Treatment
Preventive Chemotherapy Induced Peripheral Neuropathy treatments aim to reduce CIPN occurrence or severity during neurotoxic treatments. These Chemotherapy Induced Peripheral Neuropathy medications need to lessen nerve damage without affecting the cancer treatment's ability to fight tumors. While early studies in animals show that cancer-fighting abilities are preserved, large human studies are needed to confirm this. However, trials with different treatments, like drugs and vitamins, face difficulties in how they're set up. The absence of a consistent method to measure nerve damage in these trials contributes to the lack of approved drugs on the market.
Chemotherapy Induced Peripheral Neuropathy Epidemiology
The Chemotherapy Induced Peripheral Neuropathy epidemiology chapter in the report provides historical as well as forecasted data in the 7MM covering the United States, EU4 countries (Germany, France, Italy, and Spain), the United Kingdom, and Japan from 2024 to 2034. The CIPN epidemiology is segmented with detailed insights into Total Incident Population, Severity-specific Incident Population, Incident Population by Chemotherapeutic Agents, and Incident Population by Cancer Type of CIPN.
Chemotherapy Induced Peripheral Neuropathy Epidemiological Analysis
- The United States showed the highest Chemotherapy Induced Peripheral Neuropathy Incident Population accounting for approximately 41% of total cases in the 7MM.
- As per DelveInsight’s estimates, the overall incidence of moderate CIPN was maximum, followed by mild and then severe, and is subjected to increase rapidly in the coming years.
- In the US, ~93,000 breast cancer patients developed CIPN, whereas other types of cancer (excluding lung, colorectal cancer, pancreatic cancer, and multiple myeloma cancer) patients represented more than 450,000 cases of CIPN.
- Among EU4 and the UK, Germany had the highest incident population in 2023, accounting for ~28% of the total incident population of CIPN.
Chemotherapy Induced Peripheral Neuropathy Epidemiology Segmentation
- Total Chemotherapy Induced Peripheral Neuropathy Incident Population
- Chemotherapy Induced Peripheral Neuropathy Severity-Specific Incident Population
- Chemotherapy Induced Peripheral Neuropathy Incident Population by Chemotherapeutic Agents
- Chemotherapy Induced Peripheral Neuropathy Incident Population by Cancer Type
Chemotherapy Induced Peripheral Neuropathy Drug Analysis
The drug chapter segment of the Chemotherapy Induced Peripheral Neuropathy treatment market report encloses a detailed analysis of Chemotherapy Induced Peripheral Neuropathy marketed drugs and late-stage (Phase III and Phase II) Chemotherapy Induced Peripheral Neuropathy pipeline drugs. It also deep dives into the pivotal Chemotherapy Induced Peripheral Neuropathy clinical trials details, recent and expected market approvals, patent details, the latest news, and recent deals and collaborations.
Chemotherapy-Induced Peripheral Neuropathy Emerging Drugs
-
HALNEURON (tetrodotoxin or TTX): WEX Pharmaceuticals
Tetrodotoxin (TTX) blocks the voltage-gated sodium channels (VGSCs) found on nerves that conduct pain impulses, which are known to be affected in chronic pain conditions. Halneuron is an injectable formulation of tetrodotoxin, a novel small molecule with action on the peripheral nervous system.
WEX is in process of launching its pivotal Phase III trial for Halneuron to treat CINP.
-
ATX01: AlgoTx
ATX01 is a first-in-class topical treatment for peripheral neuropathic pain. Its development focus is on the pain of CIPN and erythromelalgia. ATX01’s pharmacological paradigm targets the epidermis and dermis where the damaged nerve fibers sit and avoids significant systemic absorption and its cascade of side effects.
The drug is in Phase II currently and In June 2022, the US FDA granted Fast Track Designation (FTD) to ATX01 for the treatment of CIPN.
Chemotherapy-Induced Peripheral Neuropathy Market Outlook
Key Chemotherapy Induced Peripheral Neuropathy Companies, such as MediciNova, Toray Industries, Asahi Kasei, AlgoTx, Trevena, Bexion Pharmaceuticals and others are evaluating their lead candidates in different stages of clinical development, respectively. They aim to investigate their products for the Chemotherapy Induced Peripheral Neuropathy treatment.
- The Chemotherapy Induced Peripheral Neuropathy Market Size was ~USD 910 million in the US in 2023 and is expected to show positive growth, mainly attributed to the increased incidence of cancer and the launch of upcoming therapy during the forecast period.
- Among available therapies, highest Chemotherapy Induced Peripheral Neuropathy Market revenue was generated by opioid analgesics which was around 50% of total revenue in 2023.
- Among E4 and the UK, Germany accounted for the highest Chemotherapy Induced Peripheral Neuropathy Market Size in 2023.
- The projected launch of HALNEURON (tetrodotoxin) by 2029 will further fuel growth of the Chemotherapy Induced Peripheral Neuropathy Market in 7MM.
Chemotherapy Induced Peripheral Neuropathy Drugs Uptake
This section focuses on the uptake rate of potential Chemotherapy Induced Peripheral Neuropathy drugs expected to be launched in the market during 2024–2034, which depends on the competitive landscape, safety, and efficacy data along with order of entry. It is important to understand that the key Chemotherapy Induced Peripheral Neuropathy companies evaluating their novel therapies in the pivotal and confirmatory trials should remain vigilant when selecting appropriate comparators to stand the greatest chance of a positive opinion from regulatory bodies, leading to approval, smooth launch, and rapid uptake.
Chemotherapy Induced Peripheral Neuropathy Pipeline Development Activities
The Chemotherapy Induced Peripheral Neuropathy therapeutics market report provides insights into different therapeutic candidates in Phase III and Phase II stages. It also analyzes key Chemotherapy Induced Peripheral Neuropathy Companies involved in developing targeted therapeutics. The Chemotherapy Induced Peripheral Neuropathy therapeutics market report covers information on collaborations, acquisitions and mergers, licensing, and patent details for Chemotherapy Induced Peripheral Neuropathy emerging therapies.
Latest KOL Views on Chemotherapy-Induced Peripheral Neuropathy
To keep up with the real-world scenario in current and emerging market trends, we take opinions from Key Industry leaders working in the domain through primary research to fill the data gaps and validate our secondary research. Industry Experts were contacted for insights on the evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake along with challenges related to accessibility.
DelveInsight’s analysts connected with 10+ KOLs to gather insights; however, interviews were conducted with 5+ KOLs in the 7MM. Centers such as University of Texas, Kyushu University Hospital, Regis University School of Pharmacy, etc., were contacted. Their opinion helps understand and validate current and emerging treatment patterns of CIPN. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
Chemotherapy Induced Peripheral Neuropathy Qualitative Analysis Report
We perform Qualitative and Chemotherapy Induced Peripheral Neuropathy therapeutics market Intelligence analysis using various approaches, such as SWOT analysis and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of gaps in disease diagnosis, patient awareness, physician acceptability, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. Conjoint Analysis analyzes multiple approved and emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy.
In efficacy, the trial’s primary and secondary outcome measures are evaluated; for instance, in Chemotherapy Induced Peripheral Neuropathy, one of the most important primary outcome measures is, the mean (±SD) change from baseline in average pain score. Further, the therapies’ safety is evaluated wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Chemotherapy Induced Peripheral Neuropathy Treatment Market Access and Reimbursement
Reimbursement of rare disease therapies can be limited due to lack of supporting policies and funding, challenges of high prices, lack of specific approaches to evaluating rare disease drugs given limited evidence, and payers’ concerns about budget impact. The high cost of rare disease drugs usually has a limited effect on the budget due to the small number of eligible patients being prescribed the drug. The US FDA has approved several rare disease therapies in recent years. From a patient perspective, health insurance and payer coverage guidelines surrounding rare disease treatments restrict broad access to these treatments, leaving only a small number of patients who can bypass insurance and pay for products independently.
The Chemotherapy Induced Peripheral Neuropathy drugs market report further provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of currently used therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Chemotherapy Induced Peripheral Neuropathy Treatment Market Report Scope
- The Chemotherapy Induced Peripheral Neuropathy therapeutics market report covers a segment of key events, an executive summary, descriptive overview, explaining its causes, signs and symptoms, pathogenesis, and currently available therapies.
- Comprehensive insight has been provided into the Chemotherapy Induced Peripheral Neuropathy epidemiology segments and forecasts, the future growth potential of diagnosis rate, and disease progression along with country specific treatment guidelines.
- Additionally, an all-inclusive account of both the current and Chemotherapy Induced Peripheral Neuropathy emerging therapies, along with the elaborative profiles of late-stage and prominent therapies, will have an impact on the current Chemotherapy Induced Peripheral Neuropathy treatment market landscape.
- A detailed review of the CIPN market, historical and forecasted market size, market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The report provides an edge while developing business strategies, by understanding trends, through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help in shaping and driving the 7MM Chemotherapy Induced Peripheral Neuropathy therapeutics market.
Chemotherapy Induced Peripheral Neuropathy Treatment Market Report Insights
- Patient-based Chemotherapy Induced Peripheral Neuropathy Market Forecasting
- Therapeutic Approaches
- Chemotherapy Induced Peripheral Neuropathy Pipeline Analysis
- Chemotherapy Induced Peripheral Neuropathy Market Size and Trends
- Existing and future Chemotherapy Induced Peripheral Neuropathy Therapeutics Market Opportunity
Chemotherapy Induced Peripheral Neuropathy Treatment Market Report Key Strengths
- 11 Years Chemotherapy Induced Peripheral Neuropathy Market Forecast
- 7MM Coverage
- CIPN Epidemiology Segmentation
- Inclusion of Country specific treatment guidelines
- KOL’s feedback on approved and emerging therapies
- Key Cross Competition
- Conjoint analysis
- Drugs Uptake
- Key Chemotherapy Induced Peripheral Neuropathy Market Forecast Assumptions
Chemotherapy Induced Peripheral Neuropathy Treatment Market Report Assessment
- Current Chemotherapy Induced Peripheral Neuropathy Treatment Market Practices
- Chemotherapy Induced Peripheral Neuropathy Unmet Needs
- Chemotherapy Induced Peripheral Neuropathy Pipeline Product Profiles
- Chemotherapy Induced Peripheral Neuropathy Therapeutics Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
Key Questions Answered in the Chemotherapy Induced Peripheral Neuropathy Market Report
Chemotherapy Induced Peripheral Neuropathy Market Insights
- What is the growth rate of the 7MM Chemotherapy Induced Peripheral Neuropathy treatment market?
- What was the Chemotherapy Induced Peripheral Neuropathy market size, the Chemotherapy Induced Peripheral Neuropathy market size by therapies, market share (%) distribution in 2020, and what would it look like in 2034? What are the contributing factors/key catalysts for this growth?
- Is there any unexplored patient setting that can open the window for growth in the future?
- What are the pricing variations among different geographies for approved and off-label therapies?
- How would the market drivers, barriers, and future opportunities affect the market dynamics and subsequent analysis of the associated trends?
- What are the current and emerging options for the treatment of CIPN?
- How many companies are developing therapies for the treatment of CIPN?
- What are the recent novel therapies, targets, mechanisms of action, and technologies developed to overcome the limitations of existing therapies?
- Patient/physician acceptability in terms of preferred treatment options as per real-world scenarios?
- What are the country-specific accessibility issues of expensive, recently approved therapies?
Reasons to Buy the Chemotherapy Induced Peripheral Neuropathy Treatment Market Report
- The Chemotherapy Induced Peripheral Neuropathy treatment market report will help in developing business strategies by understanding the latest trends and changing treatment dynamics driving the Chemotherapy Induced Peripheral Neuropathy Market.
- Insights on patient burden/disease Chemotherapy Induced Peripheral Neuropathy prevalence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years
- Understand the existing Chemotherapy Induced Peripheral Neuropathy market opportunities in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
- Identifying strong upcoming players in the Chemotherapy Induced Peripheral Neuropathy treatment market will help devise strategies to help get ahead of competitors.
- Detailed analysis and ranking of class-wise potential current and emerging therapies under the conjoint analysis section to provide visibility around leading classes.
- Highlights of access and reimbursement policies of approved therapies, barriers to accessibility of expensive off-label therapies, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing Chemotherapy Induced Peripheral Neuropathy treatment market so that the upcoming players can strengthen their development and launch. strategy.
Stay updated with us for Recent Articles


.jpg)
.jpg)

.jpg)




