Cystic Fibrosis Market Summary
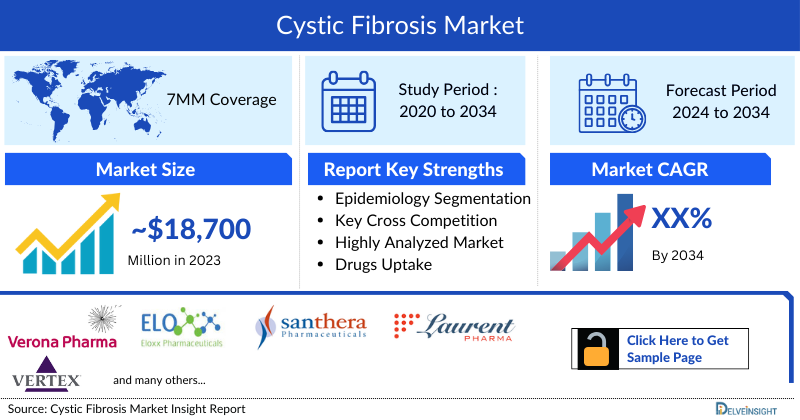
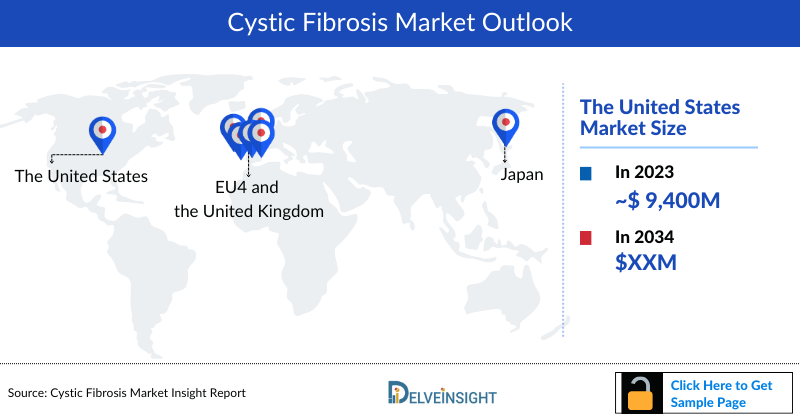
- The Cystic Fibrosis Market Size in the 7MM was approximately USD 18,743 million in 2023, out of which the US accounted for approximately USD 9,473 million.
- Cystic Fibrosis Market Size was highest in the US among the 7MM, accounting for approximately USD 7,050 million in 2023. The expected launch of potential therapies may increase the Cystic Fibrosis market size in the coming years, assisted by an increase in the prevalent population of cystic fibrosis.
Cystic Fibrosis Market and Epidemiology Analysis
- The Cystic Fibrosis Prevalence has been increasing due to factors like rising awareness among healthcare professionals and the general public, genetic influences, and shifts in healthcare policies. Additionally, as cystic fibrosis is a genetic disorder caused by mutations in the CFTR gene, there is a possibility of increased rates of consanguineous marriages or genetic predispositions, which may contribute to a rise in cystic fibrosis during the forecast period (2024-2034).
- While several Cystic Fibrosis Therapies have received approval from the US FDA, including Vertex Pharmaceuticals' TRIKAFTA, SYMDEKO, ORKAMBI, and others, none offer a cure and are often accompanied by side effects.
- Combination therapies that target multiple mutations are becoming increasingly common in the treatment of cystic fibrosis. This is because many patients have multiple mutations that contribute to the disease, and targeting multiple mutations can lead to better outcomes.
- The market growth rate for cystic fibrosis treatment could be impeded by the substantial costs associated with therapies and the absence of personalized medicines tailored to address the specific mutations underlying the disease.
- Moreover, the scarcity of qualified professionals and inadequate information will pose challenges to the cystic fibrosis market. The stringent regulatory requirements and lengthy approval processes for new therapies may also affect the Cystic Fibrosis market's growth trajectory throughout the forecast period.
- While existing Cystic Fibrosis treatments focus on managing symptoms, there is a pressing need for therapies that can slow or halt disease progression. Moreover, the development of anti-inflammatory therapies in cystic fibrosis has been challenging due to the difficulty in detecting clinical benefits, which requires studies of long duration and significant sample size.
- Despite these challenges, several therapies are being investigated for the Cystic Fibrosis therapies including Vertex Pharmaceutical’s VX-121, Verona Pharmaceutical’s Ensifentrine, and others for the management of cystic fibrosis in the 7MM.
Request for Unlocking the Sample Page of the "Cystic Fibrosis Treatment Market"
Key Factors Driving the Cystic Fibrosis Market Growth
-
Advancements in CFTR Modulator Therapies
The development of CFTR modulators, such as correctors and potentiators, has revolutionized cystic fibrosis treatment. These targeted therapies address the underlying genetic defect rather than just managing symptoms, leading to improved lung function, reduced exacerbations, and enhanced quality of life.
-
Rising Global Disease Awareness and Early Diagnosis
Increased public health initiatives, newborn screening programs, and improved diagnostic techniques have contributed to earlier detection and intervention, significantly expanding the diagnosed patient pool and driving treatment uptake.
-
Strong Research and Development Pipeline
A robust pipeline of next-generation gene and RNA-based therapies is fueling innovation in the cystic fibrosis market. These emerging treatments hold promise for addressing a wider range of genetic mutations and potentially offering curative outcomes.
-
Growing Patient Access and Reimbursement Support
Expanding reimbursement coverage for high-cost therapies and improved patient assistance programs are helping more individuals access advanced medications, contributing to overall market growth.
-
Improved Clinical Management and Multidisciplinary Care
Enhanced treatment guidelines and integrated care approaches involving pulmonologists, nutritionists, and physiotherapists have improved disease management, survival rates, and patient adherence.
DelveInsight’s report titled “Cystic Fibrosis Market Insights, Epidemiology, and Market Forecast – 2034” report delivers an in-depth understanding of burns, historical and forecasted epidemiology, as well as the burns market trends in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan. The report examines current treatment market methodologies and algorithms for cystic fibrosis, assessing the overall market potential, identifying business prospects, and addressing pertinent unmet medical requirements.
Cystic Fibrosis Treatment Market
Cystic Fibrosis is a life-limiting autosomal recessive genetic disorder that causes severe damage to the lungs and the digestive system. The disease is caused by a genetic mutation in the CFTR gene. The mutation is autosomal recessive, which implies that the gene is not on the sex chromosome, and the symptoms would manifest only when both alleles are mutated for the gene. CFTR protein is an ion channel and belongs to the ABC transporter class. Its gene is found on chromosome 7 in the long arm. It is characterized by the buildup of thick, sticky mucus in various organs, particularly the lungs and digestive system. This can lead to respiratory infections, digestive problems, and other complications.
According to the clinical spectrum of cystic fibrosis, the disease can be classified into two categories, namely, classic and non-classic cystic fibrosis. Classic cystic fibrosis: the majority of patients suffer from classic cystic fibrosis with their organs getting affected to various degrees. Patients are diagnosed with classic CF if they have one or more phenotypic characteristics and a sweat chloride concentration of >60 mmol/L; this is usually accompanied by exocrine pancreatic insufficiency (PI) or pancreatic sufficiency (PS).
The disease can have either a rapid progression of symptoms or little deterioration over time. Non-classic cystic fibrosis: This category is described by having at least one cystic fibrosis phenotype, which is accompanied by a normal (<30 mmol/L) or borderline (30–60 mmol/L) sweat chloride. They also have either single or multi-organ involvement; this is accompanied by exocrine PS and milder lung disease.
Cystic Fibrosis Diagnosis
The research and development over the last few years have contributed to the understanding of phenotypic and genotypic information about CFTR and have contributed to getting a clearer picture of the disease. Diagnosing cystic fibrosis typically involves a combination of clinical evaluation, genetic testing, and specialized tests various diagnostic tools used for cystic fibrosis diagnosis are:
a) Sweat test
It is the most reliable and widely available diagnostic test for cystic fibrosis, which involves the measurement of chloride concentration in sweat. Diagnosis with a sweat test must be made for infants with a positive NBS test. The body produces isotonic sweat in the secretory coil, but in the sweat ducts, most of the chloride is reabsorbed via the CFTR channel. Therefore, the sweat of healthy people is hypotonic. However, in individuals with dysfunctional CFTR, reabsorption of chloride does not occur, resulting in high chloride content in the sweat of people with cystic fibrosis. Pilocarpine iontophoresis is used for sweat induction which is followed by sweat collection on a gauze, filter paper (both requiring about 75 mg of sweat), or micro duct coil (requiring 50 mL). A minimum sweat rate of 1 g/m2 body surface area/min is required so that the collection time typically takes around 30 min. The sweat test is versatile in the sense that it can be performed in 2 weeks old who are normally hydrated and not acutely ill adult individuals. Patients with cystic fibrosis would have a sweat chloride concentration above 60 mmol/L.
Cystic Fibrosis Treatment
Cystic fibrosis treatment focuses on maintaining lung function by controlling respiratory infections and clearing airways of mucus, alongside administering nutritional therapy to support growth. Medications used may encompass pancreatic enzyme supplements, multivitamins (especially fat-soluble varieties), mucolytics, antibiotics (delivered via inhalation, orally, or intravenously), bronchodilators, anti-inflammatory agents, and CFTR modulators such as ivacaftor, elexacaftor, lumacaftor, and tezacaftor. These interventions collectively aim to manage complications and optimize the health and well-being of individuals with cystic fibrosis.
Cystic Fibrosis Epidemiology
As the Cystic Fibrosis market is derived using a patient-based model, the cystic fibrosis epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by total diagnosed prevalent cases of cystic fibrosis, gender-specific diagnosed prevalent cases of cystic fibrosis, age-specific diagnosed prevalent cases of cystic fibrosis, and type-specific diagnosed prevalent cases of cystic fibrosis in the 7MM covering the US, EU4 countries (Germany, France, Italy, and Spain) and the United Kingdom, and Japan from 2020 to 2034.
Key Findings from the Cystic Fibrosis Epidemiology Analysis and Forecast
- As per DeveInsight analysis, in 2023, the estimated Cystic Fibrosis Diagnosed Prevalence Cases in the 7MM were approximately 67,897 cases in 2023. This expanded genetic testing will facilitate the identification of more individuals with cystic fibrosis, contributing to the overall increase in reported cases.
- In 2023, among the 7MM, the US accounted for the highest diagnosed prevalent cases of cystic fibrosis with approximately 33,391 cases. These cases are expected to increase during the forecast period (2024-2034).
- Among the EU4 and the UK, the UK had the highest diagnosed prevalent cases of cystic fibrosis with nearly 11,275 cases, followed by France, and Germany with approximately 7,506, and 7,043 diagnosed prevalent cases respectively.
- According to DeveInsight analysis, males accounted for more diagnosed prevalent cases of cystic fibrosis than females. In 2023, there were approximately 17,363 males and 16,027 females diagnosed with prevalent cases of cystic fibrosis in the US.
- In the epidemiological model of cystic fibrosis, cases are categorized into age groups, namely children and adults. Within the EU4 and the UK, a larger proportion of cases are reported among adults, with approximately 14,651 cases observed in children and 19,801 cases in adults.
- In 2023, Japan reported an estimated 54 diagnosed prevalent cases of cystic fibrosis. Among these cases, there were approximately 2 cases of homozygous F508del, 2 cases of heterozygous F508del, and 50 cases of other mutation type-specific diagnosed prevalent cases of cystic fibrosis. These cases are expected to increase with the improved diagnosis and screening of the disease during the forecast period (2024-2034).
Cystic Fibrosis Market Recent Developments and Breakthroughs
- In March 2025, ReCode Therapeutics announced that the FDA granted Orphan Drug Designation for RCT2100, an investigational mRNA therapy developed to treat cystic fibrosis (CF).
- In February 2025, Porosome Therapeutics announced that the FDA granted Orphan Drug Designation to its revolutionary cystic fibrosis therapy, marking a significant step in secretory defect therapeutics.
- In January 2025, Lupin Limited announced that it has received tentative approval from the U.S. Food and Drug Administration (FDA) for its Abbreviated New Drug Application (ANDA) for Ivacaftor Oral Granules in 25 mg, 50 mg, and 75 mg per unit dose packet. This product is a generic version of Kalydeco Oral Granules by Vertex Pharmaceuticals.
- In January 2025, the U.S. FDA approved a new triple-combination CFTR modulator therapy—vanzacaftor, tezacaftor, and deutivacaftor. Developed by Vertex Pharmaceuticals and marketed as Alyftrek, the therapy is indicated for cystic fibrosis (CF) in patients aged 6 and older with at least one copy of a mutation responsive to the treatment, including the F508del mutation.
Cystic Fibrosis Drugs Analysis
The drug chapter segment of the cystic fibrosis treatment market report encloses a detailed analysis of cystic fibrosis-marketed drugs and late-stage (Phase III and Phase II) Cystic Fibrosis pipeline drugs. It also helps understand the cystic fibrosis clinical trials details, expressive pharmacological action, agreements and collaborations, approval and patent details, advantages and disadvantages of each included drug, and the latest Cystic Fibrosis news and press releases.
Cystic Fibrosis Marketed Drugs
-
TRIKAFTA: Vertex Pharmaceuticals
TRIKAFTA is a combination of ivacaftor—a CFTR potentiator—tezacaftor, and elexacaftor indicated for the treatment of cystic fibrosis (CF) in patients aged 12 years and older who have at least one F508del mutation in the CFTR gene. If the patient’s genotype is unknown, an FDA-cleared CF mutation test should be used to confirm the presence of at least one F508del mutation.
Elexacaftor and tezacaftor bind to different sites on the CFTR protein and have an additive effect in facilitating the cellular processing and trafficking of F508del-CFTR to increase the amount of CFTR protein delivered to the cell surface compared to either molecule alone. Ivacaftor potentiates the channel open probability (or gating) of the CFTR protein at the cell surface. Furthermore, the combined effect of elexacaftor, tezacaftor, and ivacaftor increases the quantity and function of F508del-CFTR at the cell surface, resulting in increased CFTR activity as measured by CFTR-mediated chloride transport.
Trikafta is the first approved treatment that is effective for cystic fibrosis patients 12 years and older with at least one F508del mutation, which affects 90% of the population with cystic fibrosis or roughly 27,000 people in the US.
-
SYMDEKO: Vertex Pharmaceuticals
SYMDEKO (Symkevi) is a combination of tezacaftor and ivacaftor, indicated for the treatment of patients with cystic fibrosis ages 6 years and older who are homozygous for the F508del mutation or who have at least one mutation in the CFTR gene that is responsive to tezacaftor/ivacaftor based on in vitro data and/or clinical evidence. If the patient’s genotype is unknown, an FDA-cleared cystic fibrosis mutation test should be used to detect the presence of a CFTR mutation followed by verification with bi-directional sequencing. Tezacaftor facilitates the cellular processing and trafficking of normal and select mutant forms of CFTR (including F508del-CFTR) to increase the amount of mature CFTR protein delivered to the cell surface. Ivacaftor is a CFTR potentiator that facilitates increased chloride transport by potentiating the channel-open probability (or gating) of the CFTR protein at the cell surface. For ivacaftor to function CFTR protein must be present at the cell surface. Ivacaftor can potentiate the CFTR protein delivered to the cell surface by tezacaftor, leading to a further enhancement of chloride transport than either agent alone. The combined effect of tezacaftor and ivacaftor is increased quantity and function of CFTR at the cell surface, resulting in increases in chloride transport.
-
KALYDECO: Vertex Pharmaceuticals
KALYDECO (ivacaftor; VX-770) is a CFTR potentiator indicated for the treatment of cystic fibrosis in patients aged 6 months and older who have one mutation in the CFTR gene that is responsive to ivacaftor based on clinical and/or in vitro assay data. If the patient’s genotype is unknown, an FDA-cleared CF mutation test should be used to detect the presence of a CFTR mutation followed by verification with bi-directional sequencing when recommended by the mutation test instructions for use. Ivacaftor is a potentiator of the CFTR protein. The CFTR protein is a chloride channel present at the surface of epithelial cells in multiple organs. Ivacaftor facilitates increased chloride transport by potentiating the channel open probability (or gating) of CFTR protein located at the cell surface. The overall level of ivacaftor-mediated CFTR chloride transport is dependent on the amount of CFTR protein at the cell surface and how responsive a particular mutant CFTR protein is to ivacaftor potentiation.
Note: Further marketed drugs and their details will be provided in the report…
Cystic Fibrosis Emerging Drugs
VX-121 is an orally administered small molecule, CFTR corrector that helps fix and restore the function of defective CFTR protein. In people with certain types of mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene, the CFTR protein is not processed and cannot move through the cell normally. The triple combination of vanzacaftor/tezacaftor/deutivacaftor is being developed as an investigational once-daily treatment for people with CF with certain mutations in the CFTR gene.
The drug candidate is currently undergoing evaluation in a Phase III clinical trial. Furthermore, Vertex Pharmaceuticals is conducting trials for another potential medication, VX-522. This drug involves CFTR mRNA delivery to the lung through lipid nanoparticles, aiming to target the root cause of cystic fibrosis.
-
Ensifentrine: Verona Pharmaceuticals
Ensifentrine (RPL554) is a first-in-class, inhaled, dual inhibitor of the phosphodiesterase 3 (PDE3) and phosphodiesterase 4 (PDE4) enzymes, and Verona Pharma’s lead pipeline asset. This dual inhibition enables it to combine bronchodilator and anti-inflammatory properties in one compound, differentiating it from existing drug classes used to treat COPD, including corticosteroids, beta2-agonists, and anti-muscarinic.
The drug candidate has demonstrated positive Phase II results, ensifentrine has been shown to activate the cystic fibrosis transmembrane conductance regulator, which is beneficial in reducing mucous viscosity and improving mucociliary clearance. This suggests ensifentrine has potential as a therapy for cystic fibrosis. Additionally, ensifentrine is being investigated in non-cystic fibrosis bronchiectasis, asthma, and other respiratory diseases.
Cystic Fibrosis Drugs Market Insights
Cystic Fibrosis treatment encompasses various pharmacological options aimed at addressing the underlying genetic defect and managing associated symptoms. The use of cystic fibrosis transmembrane conductance regulator (CFTR) protein modulators is common. Potentiators, like ivacaftor (Kalydeco), enhance the function of defective CFTR protein that has reached the cell surface, thus improving chloride ion transport. Correctors, such as lumacaftor/ivacaftor (Orkambi) and tezacaftor/ivacaftor (Symdeko/Symkevi), aid in the processing and trafficking of CFTR protein to the cell surface, thereby increasing the amount of functional CFTR protein available.
Whereas, mucolytics like pulmozyme assist in thinning and loosening mucus within the airways, facilitating its clearance from the lungs. Bronchodilators, such as albuterol and formoterol, work by relaxing the muscles surrounding the airways, thereby easing breathing. Anti-inflammatory agents, such as corticosteroids and ibuprofen, aid in diminishing inflammation in the airways and lungs, potentially enhancing lung function and minimizing exacerbation.
Cystic Fibrosis Market Outlook
Cystic Fibrosis treatment typically involves a multidisciplinary approach aimed at managing symptoms, preventing complications, and improving quality of life. People with cystic fibrosis have difficulty breathing and suffer from bronchospasms. Hence bronchodilators are administered to them to relax the smooth muscles of airways and aid in respiration. Albuterol which is marketed as Ventolin, is the most commonly used broncho-dilating agent that provides selective agonistic action on beta2-adrenoceptors. These treatments include airway clearance techniques, mucus-thinning medications, bronchodilators, antibiotics for respiratory infections, nutritional support, lung transplantation in severe cases, and emerging gene-based therapies such as CFTR modulators.
Overall, the cystic fibrosis drugs market is expected to continue growing as research efforts advance and new therapies become available to meet the needs of cystic fibrosis patients. To meet the need for cystic fibrosis treatment, companies across the globe working in this area. Major Key Cystic Fibrosis companies include Vertex Pharmaceuticals, Verona Pharmaceuticals, Eloxx Pharmaceuticals, and several others are investigating their candidates for the management of cystic fibrosis in the 7MM. The fact that the research community is currently quite active gives hope that further novel medicines with maybe truly transforming potential will soon be made available to more patients. According to DelveInsight, the overall dynamics of the cystic fibrosis market are anticipated to change in the coming years owing to the expected launch of emerging therapies.
- In 2023, the Cystic Fibrosis Market Size in the 7MM was approximately USD 18,743 million, out of which the US accounted for approximately USD 9,473 million.
- DelveInsight’s analysts estimate that the Cystic Fibrosis drugs market is expected to show positive growth, mainly attributed to the increase in population, increasing screen time usage leading to eye disorders like cystic fibrosis and also, and the launch of upcoming therapies during the forecast period (2024-2034).
- The cystic fibrosis market size in the EU4 and the UK was nearly USD 7,395 million and accounted for nearly 39.4% of the total 7MM market size in 2023.
- Among the EU4 and the UK, Germany holds the highest Cystic Fibrosis market size of around USD 1,977 million followed by Italy, and France with approximately USD 1,505 million, and USD 1,436 million respectively. These numbers are expected to change during the forecast period.
- The cystic fibrosis Therapeutics Market Size in Japan accounted for nearly 10% of the total 7MM Cystic Fibrosis market size in 2023, these numbers are expected to change by 2034.
Cystic Fibrosis Drug Uptake
This section focuses on the uptake rate of potential Cystic Fibrosis drugs expected to be launched in the market during 2020–2034. For example, VX-121, an orally administered CFTR corrector aims to repair and enhance the function of faulty CFTR protein to a greater extent compared to existing Vertex CFTR modulators, potentially leading to improved clinical outcomes. This triple combination therapy has received Fast Track and Orphan Drug Designations from the US FDA, providing it with regulatory advantages and positioning it as a highly promising treatment option in the market.
Cystic Fibrosis Pipeline Development Activities
The Cystic Fibrosis therapeutics market report provides insights into different therapeutic candidates in Phase III, Phase II, and Phase I. It also analyzes key Cystic Fibrosis companies involved in developing targeted therapeutics. The Cystic Fibrosis pipeline segment covers information on collaborations, acquisitions and mergers, licensing, and patent details for Cystic Fibrosis emerging therapies.
Latest KOL Views on Cystic Fibrosis
To keep up with current Cystic Fibrosis Market Trends, we take KOLs and SMEs’ opinions working in the domain through primary research to fill the data gaps and validate our secondary research. Industry Experts contacted for insights on cystic fibrosis evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake, along with challenges related to accessibility, including Medical/scientific writers, Medical Professionals, Professors, Directors, and Others.
DelveInsight’s analysts connected with 50+ KOLs to gather insights; however, interviews were conducted with 15+ KOLs in the 7MM. Centers like the Medical University of South Carolina, Children’s Hospital of Richmond, University of Edinburgh, Imperial College London, Hospital San Carlo, Universität Bonn, and Nagoya City University Graduate School of Medical Sciences, were contacted. Their opinion helps understand and validate current and emerging therapy treatment patterns or cystic fibrosis Therapeutics Market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the Cystic Fibrosis Therapeutics Market and the unmet needs.
Physician’s View
According to our primary research analysis, despite considerable progress in cystic fibrosis treatment one of the primary challenges associated with cystic fibrosis, according to physicians, is the progressive decline in lung function. Cystic fibrosis is a complex and heterogeneous disease, with significant variability in disease severity and progression among patients. Physicians emphasize the need for personalized treatment approaches tailored to individual patient characteristics, including genotype, phenotype, and disease stage.
According to a KOL in the US, even though numerous innovative treatments are currently undergoing preclinical and clinical testing, some limiting factors such as mutation class, genetic profile, drug interactions, adverse effects, and cost remain the major areas of concern. Along with this, safe and efficient disease-modifying agents are still required in the treatment paradigm which serves as the cure for the disease.
As per another KOL, in the past, cystic fibrosis was a digestive and lung condition that mostly affected young children. However, in recent years, the condition has been seen to be causing multi-system complications in adults. The disease is now affecting more adults as compared to children due to a decline in its mortality rate. In another KOL in Japan, there are high numbers of cystic fibrosis patients with F508del mutation in the American or European population but CFTR mutations in the Japanese population are somewhat different where F508 variants are observed infrequently.
Cystic Fibrosis Qualitative Analysis
We perform Qualitative and cystic fibrosis therapeutics market Intelligence analysis using various approaches, such as SWOT analysis and Attribute Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the Analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving treatment landscape.
Conjoint Analysis analyzes multiple emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy. In efficacy, the trial’s primary and secondary outcome measures are evaluated; for instance, in cystic fibrosis trials, one of the most important primary outcome measures is complete eschar removal.
Further, the therapies’ safety is evaluated wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the route of administration, order of entry and designation, probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Cystic Fibrosis Therapeutics Market Access and Reimbursement
The high cost of therapies for the Cystic Fibrosis treatment is a major factor restraining the growth of the drug market. Because of the high cost, the economic burden is increasing, leading the patient to escape from proper treatment. The reimbursement challenges related to medical care and treatment for individuals with cystic fibrosis can be significant as it often requires specialized medical attention, covering the costs of diagnosis, treatment, and ongoing care.
Health insurance plans may not fully cover limited coverage of some medical treatments, and therapies specific to cervical dystonia. This can result in high out-of-pocket expenses for families seeking the best care for their loved ones. Moreover, it requires specialized care from healthcare providers with expertise. Finding and accessing such specialists may be challenging, and the associated costs may not always be fully reimbursed by insurance.
Cystic Fibrosis Therapeutics Market Report Scope
- The Cystic Fibrosis therapeutics market report covers a segment of key events, an executive summary, descriptive overview, explaining its causes, signs and symptoms, pathogenesis, and currently available therapies.
- Comprehensive insight has been provided into the Cystic Fibrosis epidemiology segments and forecasts, the future growth potential of diagnosis rate, disease progression, and treatment guidelines.
- Additionally, an all-inclusive account of the current and emerging therapies, along with the elaborative profiles of late-stage and prominent therapies, will impact the current treatment landscape.
- A detailed review of the cystic fibrosis therapeutics market, historical and forecasted Cystic Fibrosis treatment market size, Cystic Fibrosis drugs market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The Cystic Fibrosis therapeutics market report provides an edge while developing business strategies, by understanding trends, through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help shape and drive the 7MM cystic fibrosis treatment market.
Cystic Fibrosis Therapeutics Market Report Insights
- Patient-based Cystic Fibrosis Market Forecasting
- Cystic Fibrosis Therapeutic Approaches
- Cystic Fibrosis Pipeline Drugs Analysis
- Cystic Fibrosis Market Size
- Cystic Fibrosis Market Trends
- Existing and Future Cystic Fibrosis Drugs Market Opportunity
Cystic Fibrosis Therapeutics Market Report Key Strengths
- 11-year Cystic Fibrosis Market Forecast
- The 7MM Coverage
- Cystic Fibrosis Epidemiology Segmentation
- Key Cross Competition
- Attribute analysis
- Cystic Fibrosis Drugs Uptake
- Key Cystic Fibrosis Market Forecast Assumptions
Cystic Fibrosis Treatment Market Report Assessment
- Current Cystic Fibrosis Treatment Market Practices
- Cystic Fibrosis Unmet Needs
- Cystic Fibrosis Pipeline Drugs Analysis Profiles
- Cystic Fibrosis Drugs Market Attractiveness
- Qualitative Analysis (SWOT and Attribute Analysis)
Key Questions Answered in Cystic Fibrosis Market Report
Cystic Fibrosis Treatment Market Insights
- What was the cystic fibrosis drugs market share (%) distribution in 2020 and what it would look like in 2034?
- What was the total cystic fibrosis market size by therapies, and Cystic Fibrosis market share (%) distribution in 2020, and what would it look like by 2034? What are the contributing factors for this growth?
- How will Ensifentrine and VX-121 affect the treatment paradigm of cystic fibrosis?
- Which Cystic Fibrosis drug is going to be the largest contributor by 2034?
- What are the pricing variations among different geographies for approved and marketed therapies?
- How would future opportunities affect the Cystic Fibrosis market dynamics and subsequent analysis of the associated trends?
Cystic Fibrosis Epidemiology Insights
- What are the disease risks, burdens, and cystic fibrosis unmet needs? What will be the growth opportunities across the 7MM concerning the cystic fibrosis patient population?
- What is the historical and forecasted cystic fibrosis patient pool in the United States, EU4 (Germany, France, Italy, and Spain) the United Kingdom, and Japan?
- Out of the above-mentioned countries, which country would have the highest diagnosed prevalent cystic fibrosis population during the forecast period (2023–2034)?
- What factors are factors contributing to the growth of cystic fibrosis cases?
Current Cystic Fibrosis Treatment Market Scenario, Marketed Drugs, and Emerging Therapies
- What are the current options for the Cystic Fibrosis treatment? What are the current guidelines for treating cystic fibrosis in the US and Europe?
- How many cystic fibrosis companies are developing therapies for the cystic fibrosis treatment?
- How many emerging therapies are in the mid-stage and late stage of development for treating cystic fibrosis?
- What are the recent novel therapies, targets, Cystic Fibrosis mechanisms of action, and technologies developed to overcome the limitations of existing therapies?
- What is the cost burden of current treatment on the patient?
- Patient acceptability in terms of preferred treatment options as per real-world scenarios?
- What are the country-specific accessibility issues of approved therapies?
- What is the 7MM historical and forecasted cystic fibrosis drug market?
Reasons to Buy the Cystic Fibrosis Market Report
- The Cystic Fibrosis market size report will help develop business strategies by understanding the latest trends and changing treatment dynamics driving the cystic fibrosis drug market.
- Insights on patient burden/disease cystic fibrosis prevalence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years.
- Understand the existing Cystic Fibrosis drug market opportunities in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data in the US, EU4 (Germany, France, Italy, and Spain) the United Kingdom, and Japan.
- Identifying strong upcoming Cystic Fibrosis companies in the market will help devise strategies to help get ahead of competitors.
- Detailed analysis and ranking of class-wise potential current and emerging therapies under the conjoint analysis section to provide visibility around leading classes.
- Highlights of access and reimbursement policies for stuttering, barriers to accessibility of approved therapy, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the Cystic Fibrosis unmet needs of the existing cystic fibrosis therapeutics market so that the upcoming players can strengthen their development and launch strategy.
Stay Updated with us for New Articles:-
- Is Bacteriophage a Potential Game-Changer in the Realm of Cystic Fibrosis Treatment?
- Non-Cystic Fibrosis Bronchiectasis (NCFB) Treatment: The Quest for Effective Therapies
- Cystic Fibrosis Market: A Rapidly Expanding Playing Field for Mid-Cap Pharma
- Cystic Fibrosis Drug Market: Emerging drugs that may improve lung infections
- Approach for Cystic Fibrosis; An alternative for Statins; Oral medications not safe; Carbon monoxide poisoning treatment gets approval
- Non-cystic Fibrosis Bronchiectasis Market Infographics
- Cystic Fibrosis Market: Infographics
- Cystic Fibrosis Newsletter
- Latest DelveInsight Blogs




-market.png&w=256&q=75)

