Non-Cystic Fibrosis Bronchiectasis (NCFB) Treatment: The Quest for Effective Therapies
Apr 24, 2023
Table of Contents
Bronchiectasis is a chronic respiratory condition where the bronchial tubes in the lungs become damaged, inflamed, and widened, leading to a buildup of mucus that makes breathing difficult. The condition can develop for various reasons, including infections, genetic disorders, autoimmune conditions, and other factors.
Cystic fibrosis bronchiectasis is associated with a genetically inherited cystic fibrosis disorder caused by a CFTR gene mutation. This results in the production of thick, sticky mucus that can block the airways and lead to lung infections.
Downloads
Click Here To Get the Article in PDF
Most bronchiectasis cases – the non-cystic fibrosis bronchiectasis (NCFB)- as the name suggests, are not due to cystic fibrosis and are mostly associated with recurrent lung infections, autoimmune conditions, chronic obstructive pulmonary disease (COPD), and other lung conditions. NCFB can also develop due to inhaling toxic substances like chemicals or smoke.
Uncovering the rising hidden burden
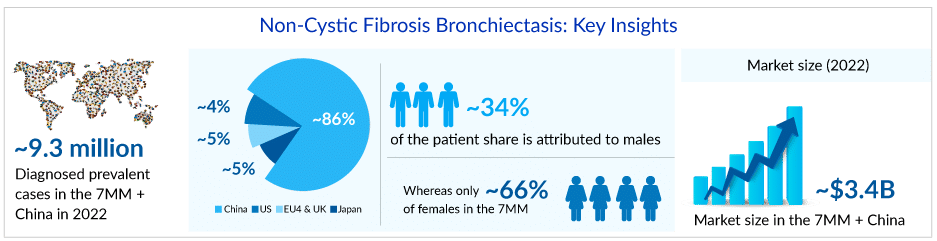
Non-cystic fibrosis bronchiectasis prevalence has been rising rapidly over the past decade, possibly as a result of increased disease awareness among healthcare providers, the availability of improved diagnostic tools such as high-resolution computed tomography (HRCT), improved longevity of older adults, the emerging epidemic of comorbidities and chronic lung diseases like chronic obstructive pulmonary disease (COPD) and asthma. DelveInsight estimates that the diagnosed prevalent cases of NCFB in the US were around 350K in 2022.
NCFB can occur in people of all ages, but it is most commonly diagnosed in middle-aged and older adults. Studies have shown that the prevalence of NCFB increases with age, with the highest rates of the condition observed in individuals over the age of 60, contributing to more than 60% of NCFB cases. However, NCFB can also affect younger individuals, particularly those with underlying lung conditions or a history of recurrent lung infections.
As per Delveinsight’s analysis, idiopathic and infection-related bronchiectasis represent the majority of adult cases of NCFB. The mucus-filled airways in patients with bronchiectasis foster the growth of various organisms. The most commonly associated pathogens to the disease include P. aeruginosa, H. influenzae, Streptococcus, and Staphylococcus. Non-tuberculous mycobacterium, viral and fungal infections are also responsible for exacerbations. P. aeruginosa is the most prevalent pathogen isolated from the sputum of approximately 30% of patients with NCFB.
Smoking, female sex, diabetes, emergency room visits, use of oral antibiotics due to exacerbation, lack of respiratory physiotherapy, absence of vaccination against pneumococci, limited mobility, and other factors are correlated with mortality risk in patients with NCFB. Early recognition of these factors and effective treatments may improve care management and quality of life.

A Call for non-cystic fibrosis bronchiectasis treatment improvement
There is currently no approved cure for NCFB. Disease management includes symptomatic treatments and prevention of respiratory infections and exacerbations. While there is some variation in the views of key opinion leaders and leading physicians on a defined non-cystic fibrosis bronchiectasis treatment pattern, there is a consensus on the importance of a multidisciplinary approach.
“Some off-label medications available for NCFB patients only provide relief from symptoms and help manage frequent infections. Some pharmacological interventions are costly, have unwanted side effects, and may require a significant time commitment, such as nebulization therapies. Non-pharmacological interventions, like Chest physiotherapy (CPT), need to be performed on a long-term basis with the patient’s adherence to treatment to yield desired effects.” -Key Opinion Leader
A current non-cystic fibrosis bronchiectasis treatment may involve antibiotics to control infections, airway clearance techniques to help remove mucus from the lungs, bronchodilators to help relax the muscles in the airways and make breathing easier, and corticosteroids and other medications to help reduce inflammation in the airways. Patients with severe NCFB may require oxygen therapy or lung transplantation.
The antibiotic treatment associated with NCFB includes antibiotics for exacerbation, suppressive antibiotics, rotating oral suppressive antibiotics, or inhaled suppressive antibiotics. Inhaled antibiotics are generally considered effective and safer than oral and intravenous varieties; however, there are differences of opinion concerning the same. Comparative trials on inhaled antibiotics are limited and controversial due to the bias in population selection and methodological variability.
Three types of inhaled non-dry antibiotics used for NCFB patients include tobramycin, colistin, and amikacin. Colistin belongs to the polymixin class of antibacterial, whereas the other two are from the aminoglycoside class. Nevertheless, despite several encouraging pieces of evidence, the prolonged use of antibiotic prophylaxis can cause an increased risk of the emergence of antibiotic resistance that should not be underestimated.
Inhaled corticosteroids (ICS) have also been investigated in NCFB, and studies have been conducted to compare the use of high-dose ICS and medium-dose ICS. Nebulized 7% hypertonic saline is also considered safe and effective in addressing sputum retention and improving lung function.
The use of macrolides for non-cystic fibrosis bronchiectasis treatment has become common in recent years due to several characteristics, their anti-inflammatory effects, ability to decrease mucus production, and well-known effect on Gram-positive cocci and atypical pathogens. Recent evidence demonstrates that long-term treatment with macrolides, particularly azithromycin, significantly reduced the incidence of NCFB exacerbations improving quality of life.
The non-cystic fibrosis bronchiectasis treatment market faces several challenges and concerns that hinder the development of effective treatments. There are no specific NCFB therapies approved by the FDA that target the causes of NCFB, and the available off-label non-cystic fibrosis bronchiectasis treatments primarily focus on managing symptoms and preventing exacerbations. There is also a lack of globally or nationally recognized treatment guidelines. Many non-cystic fibrosis bronchiectasis treatments are based on limited evidence from small studies and observational data rather than large randomized controlled trials. This means there is uncertainty about the effectiveness and safety of these treatments. Adverse medication effects can also occur, potentially exacerbating the disease or causing new health problems. Compliance with non-cystic fibrosis bronchiectasis treatment regimens can be difficult for patients, leading to poor outcomes and increased exacerbation risks. Additionally, the cost of non-cystic fibrosis bronchiectasis treatments can be prohibitively high, limiting access to necessary care for some patients. Addressing these challenges is critical to developing effective non-cystic fibrosis bronchiectasis treatments and improving patient outcomes.
Unraveling the barricades in the development of effective non-cystic fibrosis bronchiectasis treatment
Pharmaceutical companies face several challenges in developing new non-cystic fibrosis bronchiectasis treatments. Some of these challenges include:
- Heterogeneity of the disease: NCFB is a complex and heterogeneous disease with diverse clinical presentations and underlying pathologies. This makes it difficult to identify specific therapeutic targets and develop treatments that are effective for all patients. E.g., P. aeruginosa, a common and dominant pathogen, is found in the airways of both cystic fibrosis and non-cystic fibrosis bronchiectasis patients. This limits P. aeruginosa eradication strategies for NCFB patients.
- Lack of validated endpoints: There has been no universally accepted set of endpoints for non-cystic fibrosis bronchiectasis clinical trials in the past, making it difficult for companies to compare the efficacy of treatments and assess their impact on disease progression. There have been instances where the FDA has suggested new Phase III trials to examine the frequency of pulmonary exacerbations (PE) where the initial primary endpoint analyzed was the time to first exacerbation (TFE). As per the FDA, TFE was a parsimonious endpoint and was easy to analyze, ignored all clinical events occurring after initial PE, and was less clinically relevant for patients expected to be on therapy for prolonged periods or life-long. Exacerbations are undeniably a significant cause of morbidity and, to a lesser degree, mortality in patients with bronchiectasis. However, the daily burden of cough and sputum is an equal or greater concern for many patients.
- Limited understanding of disease pathogenesis: Despite recent advances in understanding the pathogenesis of NCFB, there is still much that is not known about the underlying mechanisms that drive disease progression. This makes it difficult to identify specific targets for therapeutic intervention.
- Limited patient population: NCFB is a relatively rare disease, making it difficult for pharmaceutical companies to conduct large-scale clinical trials and develop cost-effective non-cystic fibrosis bronchiectasis treatments.
- Limited funding: Due to the relatively small patient population and lack of awareness of the disease, funding for research and development of new non-cystic fibrosis bronchiectasis treatments is limited compared to other respiratory diseases.
- Development of resistance: The emergence of drug-resistant bacteria is a major concern in developing new antibiotics for non-cystic fibrosis bronchiectasis treatment.
- Failures of clinical trials: There have been a few investigational therapies that have failed to demonstrate significant clinical benefits with regard to lung function, exacerbation frequency, or quality of life. Savara’s APULMIQ has been studied in two Phase III studies, ORBIT-3 (NCT01515007) and ORBIT-4 (NCT02104245). The US FDA rejected the drug’s application due to concerns about their clinical data, discordant trial findings, and product quality. Similarly, Aradigm Corporation was forced to withdraw its application to the EMA as it was determined that the trial data did not demonstrate a “positive benefit-risk balance.”
The complex nature of NCFB and the various obstacles encountered in creating successful therapies highlight the significance of continued research and collaboration among companies to identify and address the underlying mechanisms driving this disease.
Navigating the path to quench non-cystic fibrosis bronchiectasis treatment market’s thirst for approved products
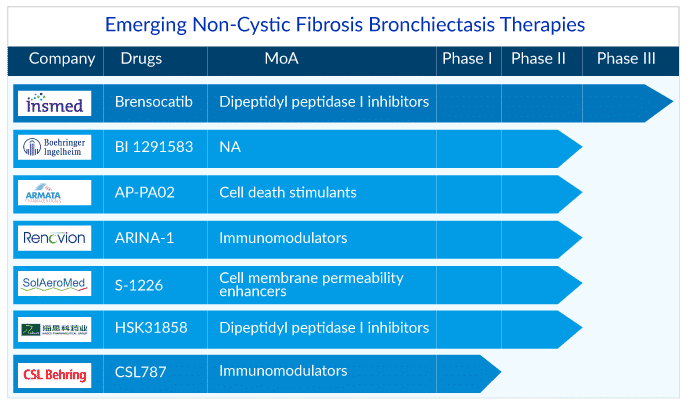
To improve the current non-cystic fibrosis bronchiectasis treatment regime, pharmaceutical companies are actively researching new treatments for NCFB. Molecules with curative capacities and novel mechanisms of action, such as reversible dipeptidyl peptidase 1 inhibitor, Cathepsin C Inhibitor (CatC) inhibitor, cell membrane permeability enhancers, and others, are being developed. Over the next couple of years, the non-cystic fibrosis bronchiectasis treatment market is expected to get a boost with the launch of potential drugs like brensocatib (formerly INS1007) (Insmed/AstraZeneca), colistimethate sodium (CMS), I-neb (Zambon), and others. Although there are not many non-cystic fibrosis bronchiectasis drugs in late stages of development, there are approximately 15 drugs in various early stages by pharma giants such as AstraZeneca, Boehringer Ingelheim, and others, and it is evident that there is a huge interest in this untapped non-cystic fibrosis bronchiectasis market.
“Precision medicine is becoming a hopeful approach to improve patient outcomes for NCFB. Endophenotypes are being explored to find common traits that may be tailored for particular treatments and interventions in individual patients. Since the NCFB patient population is heterogeneous and includes various clinical phenotypes accompanied by one or more endotypes and disease-causing mechanisms that may occur independently or together, recognizing different disease phenotypes can allow tailored treatments for patients, which could potentially improve clinical outcomes.” -Key Opinion Leader
Brensocatib is a novel oral medication that is being developed for non-cystic fibrosis bronchiectasis treatment. It is a reversible inhibitor of dipeptidyl peptidase 1 (DPP1), an enzyme that is involved in the activation of neutrophils and the production of neutrophil extracellular traps (NETs). NETs contribute to chronic inflammation and tissue damage in the lungs of patients with NCFB. Brensocatib can help reduce inflammation and prevent lung damage in patients with NCFB. The drug was well-tolerated and effectively reduced inflammation in clinical trial patients with NCFB. In Phase II clinical trials, brensocatib was shown to reduce exacerbation rates and sputum neutrophil counts and improve lung function in patients with NCFB.
Brensocatib has been granted Breakthrough Therapy designation (BTD) by the US FDA and Priority Medicines (PRIME) designation from the EMA for non-cystic fibrosis bronchiectasis treatment, which is intended to expedite the development and review of the drug. It is undergoing Phase III clinical trials to evaluate its safety and efficacy in a larger patient population. If approved, brensocatib would be the first approved drug for NCFB. As per DelveInsight analysts’ the drug might generate a revenue of approximately USD 7 million in the US in its entry year. Compared to current non-cystic fibrosis bronchiectasis treatment options, such as antibiotics and airway clearance techniques, brensocatib has several potential advantages.
- The drug targets the underlying inflammatory process that drives the progression of NCFB. This means that brensocatib has the potential to slow disease progression, which could improve long-term outcomes for patients.
- It is an oral medication, which makes it more convenient and less invasive than several current treatments, which often require nebulizers or other respiratory devices. This could improve treatment adherence and quality of life for patients.
- It has a favorable safety profile, with no serious adverse events reported in clinical trials. This suggests it could be a safe and well-tolerated treatment option for patients with NCFB.
Brensocatib is expected to face competition from other emerging treatments for NCFB, such as colistimethate sodium, which could affect its market potential. Colistimethate sodium is an antibiotic used to treat bacterial infections, including those associated with NCFB. Zambon is developing a new formulation of colistimethate sodium, delivered via inhaler, to improve its effectiveness and reduce the risk of side effects associated with systemic administration. Zambon has received US FDA BTD, Qualified Infectious Disease Product (QIDP), and Fast Track Designation (FTD) for Colistimethate sodium powder for nebulization solution (CMS I-neb) in patients with NCFB colonized with P. aeruginosa.
Colistimethate sodium (CMS) I-neb is anticipated to specifically target adult NCFB patients colonized with P. aeruginosa, unlike brensocatib, which will target the entire NCFB patient pool. The drug is estimated to attain its peak sales in its 7th year.
Other assets in development non-cystic fibrosis bronchiectasis treatment include CSL787 (CSL Behring), CHF 6333 (Chiesi Farmaceutici), BI 1291583 (Boehringer Ingelheim), AP-PA02 (Armata Pharmaceuticals), ARINA-1 (Renovion), S-1226 (SolAeroMed), among others. These early-stage products in development are expected to be more effective than the current antibiotic therapy and improve the non-cystic fibrosis bronchiectasis treatment regime in the distant future.
FAQs
DelveInsight’s epidemiology model estimates that there were ~1 million diagnosed prevalent cases of NCFB in the 7MM in 2024, which is expected to increase throughout the forecast period (2025–2034).
Currently, Insmed’s BRINSUPRI is the only approved drug for NCFB treatment. As the first DPP-1 inhibitor in respiratory care, BRINSUPRI’s distinct mechanism and first-to-market position could establish it as a standard of care for NCFB.
Some of the key non-cystic fibrosis bronchiectasis therapies in the clinical trials include Inhaled Colistimethate Sodium (CMS I-neb), ARINA-1 (RVN-301), HSK31858, AP-PA02, Ensifentrine (Nebulizer), Itepekimab, BI 1291583, CHF 6333, CSL787, XH-S004, MDI-0151, and others.
According to DelveInsight’s analysis, the total market size for NCFB across the key geographies, such as the US, EU4, the UK, and Japan, was valued at USD 1.5 billion in 2024. The US non-cystic fibrosis bronchiectasis market is expected to grow at a CAGR of 11.7% during the study period (2020–2034).

Downloads
Article in PDF