Non-Cystic Fibrosis Bronchiectasis Market
-
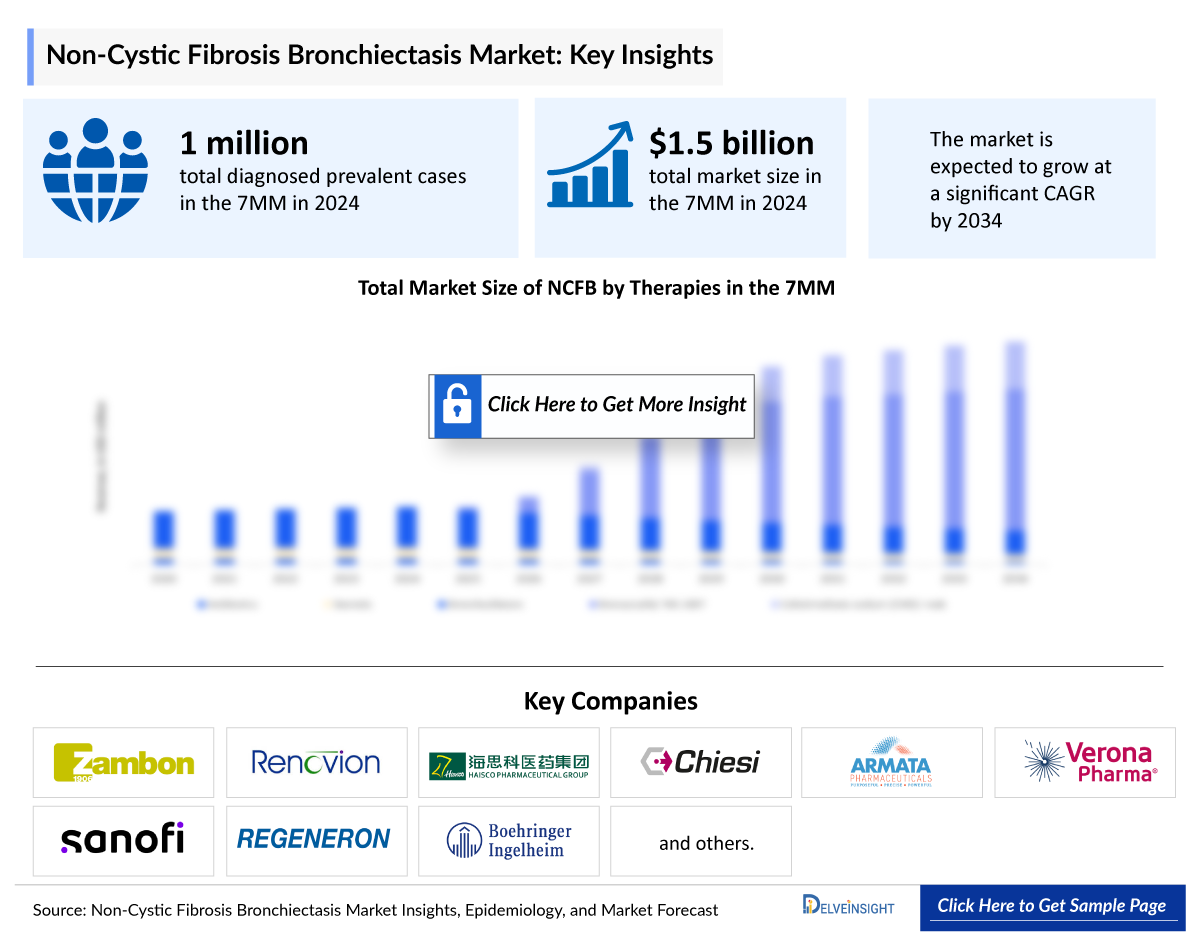
The Non-Cystic Fibrosis Bronchiectasis market in the 7MM was valued at approximately USD 1,487 million in 2025 and is expected to reach approximately USD 5,768 million in 2034. Over the forecast period from 2025 to 2034.
-
The Non-Cystic Fibrosis Bronchiectasis market is projected to grow at a CAGR of 16.25% by 2034 in leading countries (US, EU4, UK and Japan).
Non-Cystic Fibrosis Bronchiectasis Market and Epidemiology Analysis
- According to DelveInsight’s estimates, in 2023, there were approximately 1,028,651 Non Cystic Fibrosis Bronchiectasis diagnosed prevalent cases in the 7MM. Of these, the US accounted for approximately 37% of the cases, EU4 and the UK countries accounted for around 54%, followed by Japan which represented nearly 9%.
- A critical Non Cystic Fibrosis Bronchiectasis Unmet Need is the lack of approved, disease-specific therapies. Current treatments primarily focus on symptom management rather than addressing the underlying pathology, creating a significant gap in effective long-term solutions and driving demand for targeted, innovative drug development.
- Non-Cystic Fibrosis Bronchiectasis companies focus on developing advanced treatments targeting chronic airway inflammation and infection. Key players include Insmed, Aradigm Corporation, AstraZeneca, Bayer, and Grifols, actively engaged in drug development.
Non-Cystic Fibrosis Bronchiectasis Market size and forecast
- 2025 NCFB Market Size: USD 1,487 million
- 2034 Projected NCFB Market Size: USD 5,768 million
- NCFB Growth Rate (2025-2034): 16.25% CAGR
- Largest NCFB Market: United States
Request for Unlocking the Sample Page of the "Non Cystic Fibrosis Bronchiectasis Treatment Market"
DelveInsight’s “Non Cystic Fibrosis Bronchiectasis Market Insights, Epidemiology, and Market Forecast – 2034” report delivers an in-depth understanding of Non Cystic Fibrosis Bronchiectasis , historical and forecasted epidemiology, as well as the Non Cystic Fibrosis Bronchiectasis therapeutics Market Trends in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
The Non Cystic Fibrosis Bronchiectasis Treatment Market Report provides current treatment practices, emerging drugs, market share of individual therapies, and current and forecasted 7MM NCFB market size from 2020 to 2034. The report also covers Non Cystic Fibrosis Bronchiectasis Treatment Market practices/algorithms and unmet medical needs to curate the best opportunities and assess the market’s potential. report delivers an in-depth understanding of NCFB, historical and forecasted epidemiology, as well as the Non Cystic Fibrosis Bronchiectasis Market trends in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
|
Study Period |
2020 to 2034 |
|
Forecast Period |
2025-2034 |
|
Geographies Covered |
|
|
Non Cystic Fibrosis Bronchiectasis Market |
|
|
Non Cystic Fibrosis Bronchiectasiss Market Size | |
|
Non Cystic Fibrosis Bronchiectasis Companies |
Insmed, Zambon, AstraZeneca, Renovion, Sanofi, Haisco Pharmaceutical Group, Chiesi Farmaceutici S.p.A, Armata Pharmaceuticals, Verona Pharma, Regeneron Pharmaceuticals, Boehringer Ingelheim, CSL, and others. |
|
Non Cystic Fibrosis Bronchiectasis Epidemiology Segmentation |
|
Key Factors Driving the Non-Cystic Fibrosis Bronchiectasis Market
- Launch of Insmed’s BRINSUPRI
Insmed’s BRINSUPRI (brensocatib) has reached a key regulatory milestone, emerging as the first FDA-approved DPP inhibitor and the first therapy specifically approved for NCFB. As the only treatment designed exclusively for this condition, its approval represents a major scientific breakthrough, addressing a market with persistent unmet needs.
- Growing Non-Cystic Fibrosis Bronchiectasis Diagnosed Patient Pool
Recent prevalence estimates indicate that bronchiectasis is more widespread than previously recognized, with incidence increasing sharply with age. Advances in imaging and heightened awareness have contributed to higher diagnosis rates, enlarging the potential NCFB market. In 2024, approximately 390K individuals in the US were diagnosed with NCFB, while millions more are estimated to be affected globally. This figure is projected to exceed 470K by 2034.
- Non-cystic Fibrosis Bronchiectasis Competitive Landscape
Following the approval of BRINSUPRI, competition to establish a strong presence in the NCFB market has escalated. Leading companies in the space, such as Boehringer Ingelheim [Verducatib (BI 1291583) for bronchiectasis], Fosun Pharma/Expedition Therapeutics (XH-S004 for NCFB and COPD), Haisco Pharmaceutical Group/Chiesi (HSK31858 for NCFB and asthma), and Melodia Therapeutics/Alivexis (MDI-0151, currently in preclinical stages but showing potential in ANCA-associated vasculitis, bronchiectasis, COPD, and other conditions), are actively advancing their DPP1 inhibitor programs in an effort to reshape the treatment landscape.
- Other Classes of Therapies in NCFB Clinical Trials
Beyond DPP1 inhibitors such as BRINSUPRI, which have demonstrated potential in managing NCFB by targeting neutrophilic inflammation, ongoing research is investigating other types of inhibitors, including cell membrane modulators (CMS I-neb; Zambon), immunomodulators (ARINA-1; Renovion), and additional agents, to tackle this condition.
Non Cystic Fibrosis Bronchiectasis Disease Understanding
NCFB is a chronic inflammatory lung condition characterized by irreversible bronchial dilation, resulting in persistent sputum production and compromised bacterial clearance. This dysfunction establishes a detrimental cycle where ineffective pathogen elimination leads to recurrent infections, chronic inflammation, and progressive pulmonary damage. Symptoms commonly include cough, excessive sputum production, and frequent respiratory infections, alongside additional manifestations such as shortness of breath, wheezing, hemoptysis, and chest pain.
The severity of these symptoms can vary considerably among individuals, with some experiencing mild, sporadic episodes while others endure daily, debilitating symptoms, often exacerbated by infectious triggers. NCFB is frequently associated with comorbidities like anxiety, depression, and fatigue, which significantly diminish patients’ quality of life and contribute to longer hospital stays and increased outpatient visits. The etiology of NCFB is multifaceted, with a considerable proportion of cases attributed to idiopathic factors, post-infective complications, and associations with conditions such as COPD and asthma, underscoring the complexity of its management.
Non-Cystic Fibrosis Bronchiectasis Diagnosis
The diagnosis of NCFB is primarily established through chest computed tomography scans, which provide detailed imaging of bronchial dilation and associated abnormalities. Complementary diagnostic modalities, including chest X-rays, bronchoscopy, lung function tests, blood tests, and sputum cultures, are utilized to identify potential underlying conditions and assess microbial colonization. Given the heterogeneous nature of bronchiectasis and its multifactorial origins, no single diagnostic measure can comprehensively evaluate disease severity or prognosis. To address this complexity, two validated scoring systems—the Bronchiectasis Severity Index (BSI) and the FACED score—are employed to systematically assess disease severity. These tools integrate clinical and radiological parameters, enabling a more nuanced understanding of the condition and informing individualized management strategies.
Further details related to country-based variations are provided in the report…
Non-Cystic Fibrosis Bronchiectasis Treatment
The Non Cystic Fibrosis Bronchiectasis treatment is focused on symptom management, slowing the decline in lung function, and preventing exacerbations. Chest physiotherapy is a cornerstone of management due to its effectiveness and minimal side effects, while some patients derive additional benefit from specialized interventions, including regular administration of low-dose macrolides as prophylactic antibiotics. The current Non Cystic Fibrosis Bronchiectasis Treatment regimen encompasses a multifaceted approach, incorporating antibiotics, corticosteroids, bronchodilators, acid suppression medications, active mucolytic agents, and strategies to enhance bronchial hygiene. Non-Cystic Fibrosis Bronchiectasis clinical trials investigate new antibiotics, anti-inflammatory agents, and airway clearance therapies to improve patient outcomes.
Elevated neutrophil levels in the airways underscore the potential efficacy of anti-inflammatory therapies, such as corticosteroids and macrolides, in mitigating inflammation. However, the heterogeneity of NCFB necessitates a personalized treatment approach, as existing therapies can be labor-intensive and may induce side effects, contributing to rising resistance to current medications. This complexity emphasizes the critical need for novel drug development to provide more effective and streamlined treatment options tailored to individual patient profiles.
Non Cystic Fibrosis Bronchiectasis Epidemiology
As the market is derived using a patient-based model, the Non Cystic Fibrosis Bronchiectasis Epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by total Non Cystic Fibrosis Bronchiectasis diagnosed prevalent cases, Non Cystic Fibrosis Bronchiectasis severity-specific diagnosed prevalent cases, Non Cystic Fibrosis Bronchiectasis gender-specific diagnosed prevalent cases, Non Cystic Fibrosis Bronchiectasis etiology-specific diagnosed prevalent cases, and microbiology of Non Cystic Fibrosis Bronchiectasis patients in the 7MM covering the United States, EU4 countries (Germany, France, Italy, and Spain) and the United Kingdom, and Japan from 2020 to 2034.
- According to DelveInsight’s epidemiology model, in the 7MM, the total diagnosed prevalent cases of NCFB were approximately 1,028,651 in 2023. This number is anticipated to rise during the forecast period (2024-2034), driven by increased awareness and screening, along with advancements in diagnostic techniques.
- In 2023, the US accounted for the highest number of diagnosed prevalent cases of NCFB, with approximately 380,711 cases, while France accounted for the least, with only 37,576 cases.
- Among EU4 and the UK, the UK accounted for the highest number of diagnosed prevalent cases of NCFB, with approximately 224,976 cases in 2023, followed by Spain with approximately 149,236 cases, and Italy with nearly 89,584 cases.
- Among the severity-specific diagnosed prevalent cases of NCFB in EU4 and the UK in 2023, there were approximately 235,481 moderate cases, around 163,649 severe cases, and 152,230 mild cases.
- Among the gender-specific cases of NCFB in the UK in 2023, there were approximately 130,486 cases for females and around 94,490 cases for males.
- In Japan in 2023, the majority of etiology-specific diagnosed prevalent cases of NCFB were attributed to unknown or idiopathic causes, accounting for 32,837 cases.
- In 2023, Japan had approximately 96,580 diagnosed prevalent cases of NCFB.
Non Cystic Fibrosis Bronchiectasis Epidemiology Segmentation:
- Total Diagnosed Prevalent Cases of NCFB
- Severity-specific Diagnosed Prevalent Cases
- Gender-specific Diagnosed Prevalent Cases
- Etiology-specific Diagnosed Prevalent Cases
- Microbiology of NCFB Patients
Non Cystic Fibrosis Bronchiectasis Recent Developments and Breakthroughs
- In August 2025, the FDA approved Insmed’s BRINSUPRI (brensocatib), the first oral therapy for non-cystic fibrosis bronchiectasis (NCFB) in patients 12 years and older. Insmed has set the annual price at $88,000 before discounts.
- In February 2025, Insmed Incorporated announced that the FDA has accepted its New Drug Application (NDA) for brensocatib, intended for patients with non-cystic fibrosis bronchiectasis. The FDA granted Priority Review and set a target action date of August 12, 2025, under the Prescription Drug User Fee Act (PDUFA).
- In October 2024, Insmed shared positive late-breaking subgroup data from the Phase III ASPEN study of brensocatib for patients with NCFB at the CHEST 2024 Annual Meeting.
Non Cystic Fibrosis Bronchiectasis Drug Analysis
Marketed Non Cystic Fibrosis Bronchiectasis Drugs
BRINSUPRI (brensocatib): Insmed
Brensocatib, an oral small-molecule inhibitor targeting dipeptidyl peptidase 1 (DPP1), is being developed by Insmed for the treatment of bronchiectasis, CRSsNP, and other neutrophil-driven conditions. By inhibiting DPP1, brensocatib aims to reduce inflammation by blocking the activation of neutrophil serine proteases (NSPs), such as neutrophil elastase, during neutrophil formation in the bone marrow. Insmed reported positive topline results from the Phase III ASPEN study of brensocatib in patients with NCFB, leading to plans for a New Drug Application (NDA) submission to the US FDA in late 2024.
- In August 2025, the FDA approved Insmed’s Brinsupri (brensocatib), the first oral therapy for non-cystic fibrosis bronchiectasis (NCFB) in patients 12 years and older.
Emerging Non Cystic Fibrosis Bronchiectasis Drugs
-
Inhaled Colistimethate Sodium (CMS I-neb): Zambon
CMS I-neb is an investigational inhaled therapy for adults with NCFB colonized by P. aeruginosa, potentially offering a first-in-class treatment option. It uses colistimethate sodium, a prodrug of colistin, a polymyxin antibiotic targeting aerobic Gram-negative pathogens, including drug-resistant P. aeruginosa. By disrupting the bacterial cell membrane, colistin causes cell death and serves as a last-resort treatment for infections like carbapenem-resistant P. aeruginosa.
In September 2024, Zambon released the results of the Phase III PROMIS-I and PROMIS-II studies in The Lancet Respiratory Medicine journal. The Phase III PROMIS-I trial demonstrated a significant reduction in pulmonary exacerbation rates. Although the PROMIS-II trial was terminated early due to the pandemic, pre-pandemic data showed consistency with PROMIS-I outcomes. Zambon is working with regulatory authorities to expedite patient access. The US FDA has granted CMS I-neb Breakthrough Therapy Designation (BTD), as well as QIDP and Fast Track Designation (FTD).
-
FASENRA (benralizumab): AstraZeneca
FASENRA (benralizumab) is a monoclonal antibody that targets the IL-5 receptor alpha on eosinophils, facilitating the recruitment of natural killer cells to induce apoptosis, resulting in rapid and near-complete depletion of blood and tissue eosinophils in most patients. FASENRA is currently under investigation for treating adult patients with Non-Cystic Fibrosis Bronchiectasis associated with eosinophilic inflammation (NCFB + EI). According to clinicaltrials.gov, FASENRA completed Phase III clinical trials for this indication in April 2024.
Non Cystic Fibrosis Bronchiectasis Drugs Analysis
The Non Cystic Fibrosis Bronchiectasis treatment involves several drug classes tailored to manage symptoms, reduce exacerbations, and control underlying inflammation. Antibiotics, both oral and inhaled, are essential for managing chronic bacterial colonization, particularly against pathogens like P. aeruginosa. Macrolides, often used for their anti-inflammatory properties, are beneficial in reducing exacerbation frequency. Bronchodilators, including beta-agonists and anticholinergics, help alleviate airway obstruction, while corticosteroids are used to address inflammation, although their role remains limited due to potential side effects. Mucolytic agents improve mucus clearance, and emerging anti-inflammatory agents targeting neutrophilic inflammation, such as DPP1 inhibitors, represent innovative approaches. Together, these drug classes form a comprehensive yet evolving treatment landscape for NCFB, addressing its multifaceted pathophysiology. The Non-Cystic Fibrosis Bronchiectasis drugs market is growing, driven by rising prevalence and demand for effective antibiotic and anti-inflammatory treatments.
Non Cystic Fibrosis Bronchiectasis Market Outlook
The market for NCFB is poised for significant growth due to the evolving landscape of pharmacological and non-pharmacological interventions addressing the complex pathophysiology of the disease. Current therapeutic strategies encompass a range of drug classes, including antibiotics, corticosteroids, bronchodilators, and mucolytics, with inhaled antibiotics demonstrating efficacy in managing chronic bacterial infections and reducing exacerbation rates. Emerging therapies, such as Brensocatib, a Dipeptidyl Peptidase 1 (DPP1) inhibitor, and BI 1291583, a cathepsin C inhibitor, target neutrophilic inflammation through distinct mechanisms, thereby offering novel approaches to improve patient outcomes.
Additionally, investigational therapies like CMS I-neb and monoclonal antibodies such as FASENRA and Itepekimab present further options by directly targeting specific inflammatory pathways associated with eosinophilic inflammation. The incorporation of non-pharmacological approaches, particularly Airway Clearance Techniques (ACTs), complements pharmacological regimens, enhancing mucus clearance and preventing infection. However, the market faces challenges, including a lack of consensus guidelines and under-researched therapies like mucolytics and hyperosmolar agents, which may hinder optimal patient management.
Nonetheless, ongoing clinical trials and advancements in drug development highlight the potential for innovative treatments to fill existing gaps in NCFB management, ultimately improving the quality of life for patients and reducing the burden on healthcare systems. With a promising pipeline of therapies in various stages of development, the future of NCFB treatment appears increasingly optimistic, underscoring the need for continued research and clinical validation to fully realize these opportunities.
Key Non Cystic Fibrosis Bronchiectasis Companies such as Insmed, Zambon, AstraZeneca, Renovion, Sanofi, and others are evaluating their lead candidates in different stages of clinical development. They aim to investigate their products to treat NCFB.
- The Non-Cystic Fibrosis Bronchiectasis market across the 7MM was valued at around USD 1,487 million in 2025 and is anticipated to grow to approximately USD 5,768 million by 2034. During the forecast period from 2025 to 2034, the market is expected to expand at a CAGR of 16.25%, driven by key regions such as the US, EU4, UK, and Japan.
- The total Non Cystic Fibrosis Bronchiectasis Treatment Market Size in the US was approximately USD 664.3 million in 2023, accounting for approximately 46% of the total market revenue for the 7MM.
- The total Non Cystic Fibrosis Bronchiectasis Treatment Market Size in EU4 and the UK was calculated to be approximately USD 722.9 million in 2023. Among the EU4 and the UK, the UK accounted for the highest market with approximately USD 295.0 million, followed by Spain with approximately USD 195.7 million in the respective year, and Italy with nearly USD 117.5 million.
- The total Non Cystic Fibrosis Bronchiectasis Treatment Market Size in Japan was approximately USD 67.8 million in 2023.
- Among the currently used therapies, the majority of the Non Cystic Fibrosis Bronchiectasis Market Share was of Bronchodilators, with a revenue of approximately USD 1,010.8 million in 2023 among the 7MM.
Non-Cystic Fibrosis Bronchiectasis Drugs Uptake
This section focuses on the uptake rate of potential Non Cystic Fibrosis Bronchiectasis drugs expected to be launched in the market during 2020–2034. For example Brensocatib is expected to enter the US market in 2025 and is projected to have a medium-fast uptake during the forecast period.
Further detailed analysis of emerging therapies drug uptake in the report…
Non-Cystic Fibrosis Bronchiectasis Pipeline Drugs Market
The Non Cystic Fibrosis Bronchiectasis pipeline report provides insights into different Non Cystic Fibrosis Bronchiectasis clinical trials within Phase III, Phase II, and Phase I. It also analyzes key Non Cystic Fibrosis Bronchiectasis Companies involved in developing targeted therapeutics.
Non-Cystic Fibrosis Bronchiectasis Clinical Trials Activities
The Non Cystic Fibrosis Bronchiectasis clinical trials analysis report covers information on collaborations, acquisitions and mergers, licensing, and patent details for emerging therapies for NCFB.
Latest KOL Views on Non-Cystic Fibrosis Bronchiectasis
What KOLs are saying on Non Cystic Fibrosis Bronchiectasis Patient Trends?
To keep up with current Non Cystic Fibrosis Bronchiectasis market trends, we take KOLs and SMEs’ opinions working in the domain through primary research to fill the data gaps and validate our secondary research. Industry Experts contacted for insights on NCFB evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake, along with challenges related to accessibility, including Medical/scientific writers, Medical Professionals, Professors, Directors, and Others.
DelveInsight’s analysts connected with 50+ KOLs to gather insights; however, interviews were conducted with 15+ KOLs in the 7MM. Centers like the University of Texas Health Science Center, San Antonio, Harvard Medical School, Boston, US, The Freeman Hospital, Newcastle upon Tyne, and the Fukujuji Hospital, Tokyo, Japan, among others, were contacted. Their opinion helps understand and validate current and emerging therapy treatment patterns or NCFB market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the Non Cystic Fibrosis Bronchiectasis market and the unmet needs.
Non Cystic Fibrosis Bronchiectasis Physician’s View
As per the KOLs from the US, the Non Cystic Fibrosis Bronchiectasis Prevalence is rising, especially among women and the elderly, likely driven by increased physician awareness and the expanded use of CT imaging, the diagnostic gold standard. CT imaging not only facilitates the identification of underlying causes but also provides a precise assessment of the extent and severity of NCFB.
As per the KOLs from the UK, previous severe respiratory infections, allergic bronchopulmonary aspergillosis, impaired ciliary clearance, primary or secondary immunodeficiency, and other illnesses linked with bronchiectasis, such as COPD and severe asthma, are only a few of the predisposing factors that may be found. Despite following the suggestions in the guidelines, 40% of patients cannot determine the cause of their bronchiectasis, and only 13% of those cases result in a change in how the patients are managed.
As per the KOLs from Japan, surgery is most effective for symptomatic bronchiectasis, caused by a localized structural alteration in the airway in otherwise healthy people. The most common causes of airway damage include childhood infections such as TB, measles, pertussis, and post-infectious bronchiectasis.
Non Cystic Fibrosis Bronchiectasis Drugs Market: Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the Analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving Non Cystic Fibrosis Bronchiectasis treatment market landscape.
Conjoint Analysis analyzes multiple emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy. To analyze the effectiveness of these therapies, have calculated their attributed analysis by giving them scores based on their ability to improve atrial and ventricular dimension/function and ability to regulate heart rate.
Further, the therapies’ safety is evaluated wherein the adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials, which directly affects the safety of the molecule in the upcoming trials. It sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the route of administration, order of entry and designation, probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Non Cystic Fibrosis Bronchiectasis Therapeutics Market Access and Reimbursement
DelveInsight’s ‘Non Cystic Fibrosis Bronchiectasis Market Insights, Epidemiology, and Market Forecast – 2034’ report provides a descriptive overview of the market access and reimbursement scenario of NCFB. This section includes a detailed analysis of the country-wise healthcare system for each therapy, enlightening the market access, reimbursement policies, and health technology assessments.
The Non Cystic Fibrosis Bronchiectasis therapeutics market report provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenarios, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Scope of the Non Cystic Fibrosis Bronchiectasis Therapeutics Market Report
- The Non Cystic Fibrosis Bronchiectasis Therapeutics Market Report covers a segment of key events, an executive summary, and a descriptive overview explaining its causes, signs and symptoms, pathogenesis, and currently available therapies.
- Comprehensive insight into the Non Cystic Fibrosis Bronchiectasis epidemiology segments and forecasts, the future growth potential of diagnosis rate, disease progression, and treatment guidelines have been provided.
- Additionally, an all-inclusive account of the current and emerging therapies and the elaborative profiles of late-stage and prominent therapies will impact the current Non Cystic Fibrosis Bronchiectasis treatment market landscape.
- A detailed review of the Non Cystic Fibrosis Bronchiectasis drugs market, historical and forecasted Non Cystic Fibrosis Bronchiectasis treatment market size, Non Cystic Fibrosis Bronchiectasis drugs market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The Non Cystic Fibrosis Bronchiectasis Therapeutics Market Report provides an edge while developing business strategies by understanding trends through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help shape and drive the 7MM Non Cystic Fibrosis Bronchiectasis Drugs Market.
Non-Cystic Fibrosis Bronchiectasis Therapeutics Market Report Insights
- Non Cystic Fibrosis Bronchiectasis Patient-based Market Forecasting
- Non Cystic Fibrosis Bronchiectasis Therapeutics Approaches
- Non Cystic Fibrosis Bronchiectasis Pipeline Drugs Market
- Non Cystic Fibrosis Bronchiectasis Market Size and Trends
- Existing and Future Non Cystic Fibrosis Bronchiectasis Drugs Market Opportunity
Non-Cystic Fibrosis Bronchiectasis Therapeutics Market Report key strengths
- 10 years Non Cystic Fibrosis Bronchiectasis Market Forecast
- The 7MM Coverage
- Non Cystic Fibrosis Bronchiectasis Epidemiology Segmentation
- Key Cross Competition
- Attribute analysis
- Non Cystic Fibrosis Bronchiectasis Drugs Uptake
- Key Non Cystic Fibrosis Bronchiectasis Market Forecast Assumptions
Non-Cystic Fibrosis Bronchiectasis Therapeutics Market Report Assessment
- Current Non Cystic Fibrosis Bronchiectasis Treatment Market Practices
- Non Cystic Fibrosis Bronchiectasis Unmet Needs
- Non Cystic Fibrosis Bronchiectasis Pipeline Drugs Analysis Profiles
- Non Cystic Fibrosis Bronchiectasis Drugs Market Attractiveness
- Qualitative Analysis (SWOT and Attribute Analysis)
- Non Cystic Fibrosis Bronchiectasis Market Drivers
- Non Cystic Fibrosis Bronchiectasis Market Barriers
Key Questions Answered In The Non-Cystic Fibrosis Bronchiectasis Market Report:
Non Cystic Fibrosis Bronchiectasis Treatment Market Insights
- What was the total Non Cystic Fibrosis Bronchiectasis Treatment Market Size, the Non Cystic Fibrosis Bronchiectasis Treatment Market Size by therapies, and Non Cystic Fibrosis Bronchiectasis Drugs Market Share (%) distribution in 2020, and what would it look like by 2034? What are the contributing factors for this growth?
- How will Brensocatib affect the treatment paradigm of NCFB?
- Which drug is going to be the largest contributor by 2034?
- What are the pricing variations among different geographies for marketed therapies?
- How would future opportunities affect the market dynamics and subsequent analysis of the associated trends?
Non Cystic Fibrosis Bronchiectasis Epidemiology Insights
- What are the disease risks, burdens, and Non Cystic Fibrosis Bronchiectasis Unmet Needs? What will be the growth opportunities across the 7MM with respect to the patient population pertaining to NCFB?
- What is the historical and forecasted Non Cystic Fibrosis Bronchiectasis patient pool in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan?
- Out of the countries mentioned above, which country would have the highest Non Cystic Fibrosis Bronchiectasis diagnosed prevalent population during the forecast period (2024–2034)?
- What factors are contributing to the growth of Non Cystic Fibrosis Bronchiectasis cases?
Current Non Cystic Fibrosis Bronchiectasis Treatment Market Scenario, Marketed Drugs, and Emerging Therapies
- What are the current options for the Non Cystic Fibrosis Bronchiectasis Treatment? What are the current clinical and treatment guidelines for treating Non Cystic Fibrosis Bronchiectasis?
- How many Non Cystic Fibrosis Bronchiectasis companies are developing therapies for the Non Cystic Fibrosis Bronchiectasis Treatment?
- How many emerging therapies are in the mid-stage and late stage of development for treating Non Cystic Fibrosis Bronchiectasis?
- What are the recent novel therapies, targets, Non Cystic Fibrosis Bronchiectasis Mechanisms of Action, and technologies developed to overcome the limitations of existing therapies?
- What is the cost burden of current treatment on the patient?
- Patient acceptability in terms of preferred treatment options as per real-world scenarios?
- What is the 7MM historical and forecasted market of NCFB?
Reasons to Buy Non-Cystic Fibrosis Bronchiectasis Market Forecast Report
- The Non Cystic Fibrosis Bronchiectasis therapeutics market report will help develop business strategies by understanding the latest trends and changing treatment dynamics driving the Non Cystic Fibrosis Bronchiectasis drugs market.
- Insights on patient burden/disease Non Cystic Fibrosis Bronchiectasis prevalence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years.
- Understand the existing Non Cystic Fibrosis Bronchiectasis drugs market opportunities in varying geographies and the growth potential over the coming years.
- The distribution of historical and current patient share is based on real-world prescription data in the US, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
- Identifying upcoming solid players in the Non Cystic Fibrosis Bronchiectasis drugs market will help devise strategies to help get ahead of competitors.
- Detailed analysis and ranking of class-wise potential current and emerging therapies under the conjoint analysis section to provide visibility around leading classes.
- Highlights of Access and Reimbursement policies for Non Cystic Fibrosis Bronchiectasis, barriers to accessibility of approved therapy, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing market so that the upcoming players can strengthen their development and launch strategy.
Stay Updated with us for Recent Articles

-epidemiology.png&w=256&q=75)


-market.png&w=256&q=75)

