Graft Versus Host Disease Market Summary
- The Graft Versus Host Disease Market Size in the 7MM is expected to grow from USD 1,626 million in 2025 to USD 3,304 million in 2034.
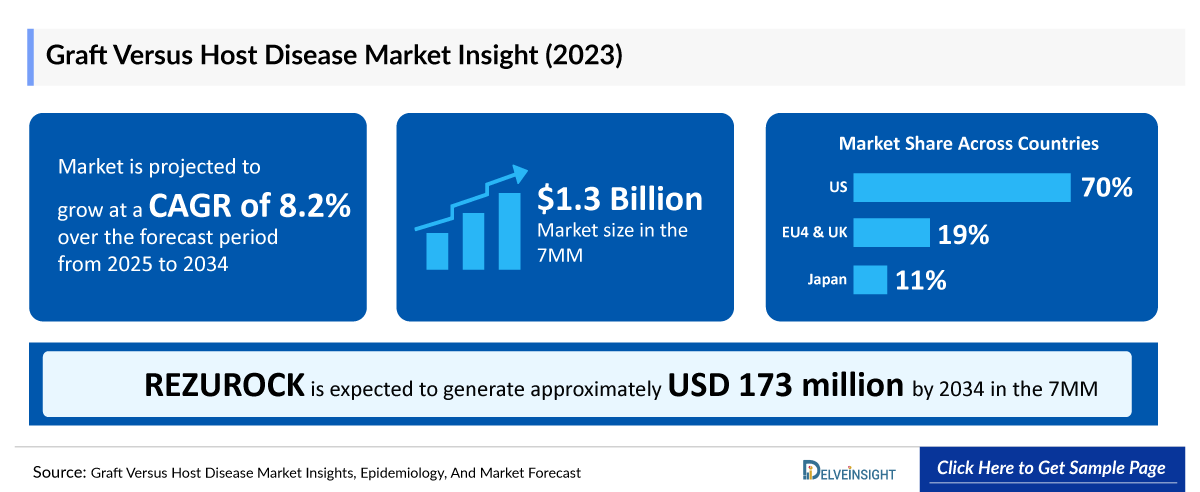
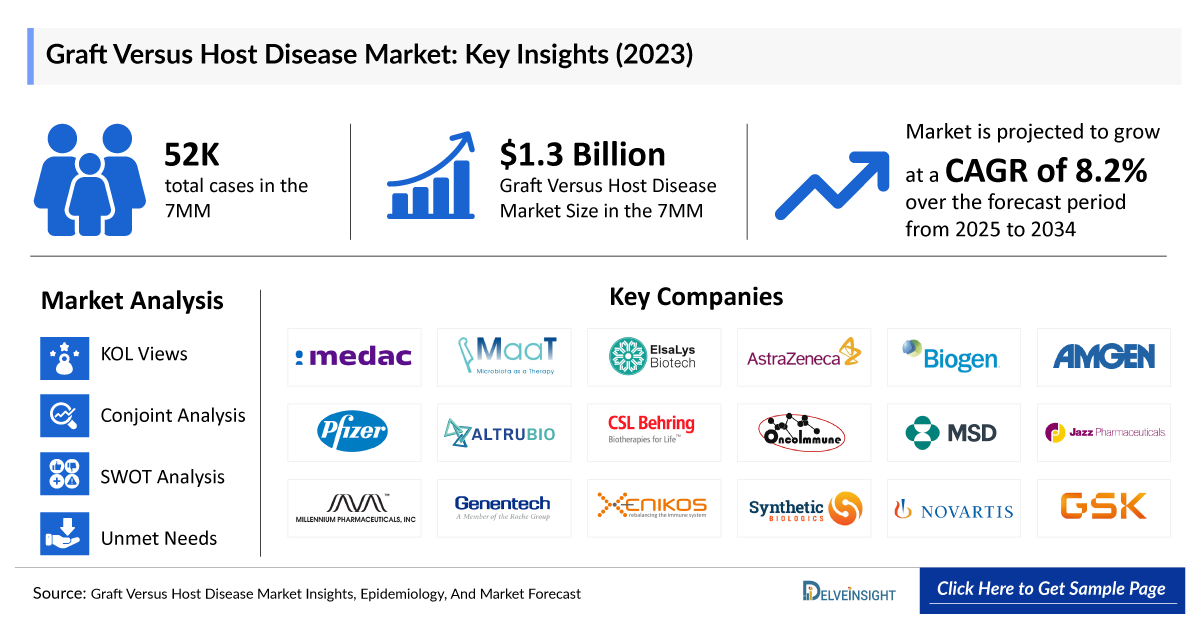
- The Graft Versus Host Disease Market is projected to grow at a CAGR of 8.2% by 2034 in leading countries like the US, EU4, UK, and Japan.
Graft Versus Host Disease Market and Epidemiology Analysis
- The Graft Versus Host Disease Treatment Market is projected to see consistent growth, with a robust compound annual growth rate (CAGR) anticipated from 2025 to 2034. This expansion across the 7MM will be driven by the introduction of innovative therapies, including ZEMAIRA, EQ001, MaaT013, and RGI-2001, among others.
- According to DelveInsight’s analysis, the Graft Versus Host Disease Market in the 7MM was valued at approximately USD 1,301.8 million in 2023.
- Graft Versus Host Disease Therapies comprising steroids, NIKTIMVO, JAKAVI/JAKAFI MOA, IMBRUVICA, RYONCIL/TEMCELL HS, and ORENCIA, among others are available in the market for managing Graft Versus Host Disease.
- Graft Versus Host Disease Companies such as CSL Behring, Equillium, MaaT Pharma, and REGiMMUNE, among others are progressing their assets through various Graft Versus Host Disease clinical trials phases, driving innovation in the Graft Versus Host Disease Market and creating significant growth opportunities.
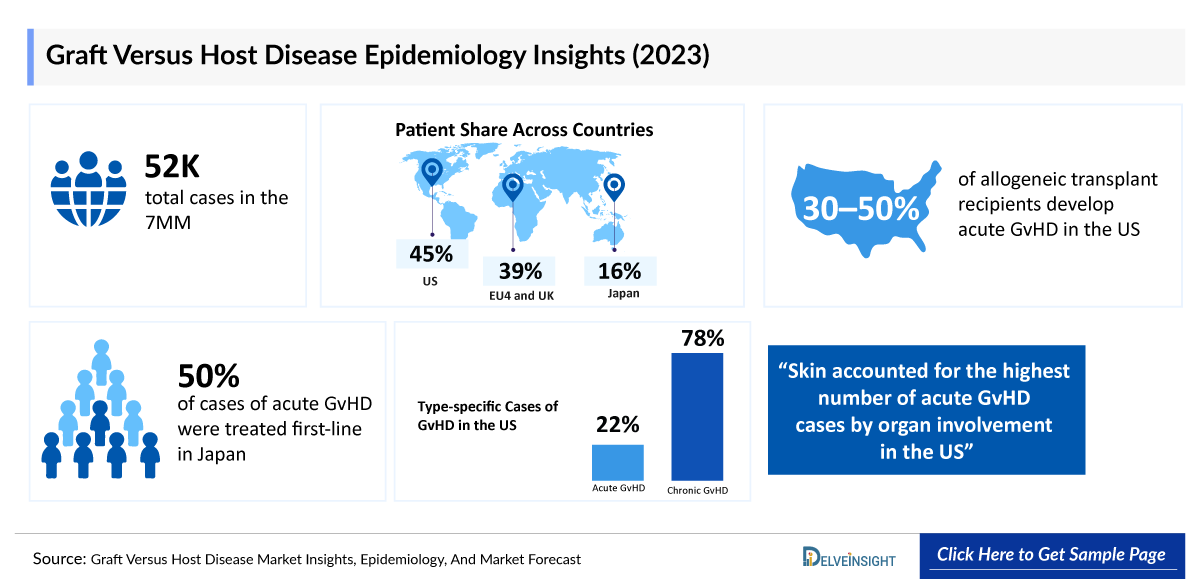
- According to DelveInsight’s estimates, in 2023, there were approximately 52 thousand cases of Graft Versus Host Disease in the 7MM. Of these, the United States accounted for 45% of the cases, while EU4 and the UK accounted for nearly 39% and Japan represented 16% of the cases, respectively.
- In November 2024, Equillium accelerated the completion timeline for EQ001 of the Phase III EQUATOR study in Graft Versus Host Disease to Q1 2025.
Graft Versus Host Disease Market Size and Forecasts
- 2025 Graft Versus Host Disease Market Size: USD 1,626 million in 2025
- 2034 Projected Graft Versus Host Disease Market Size: USD 3,304 million in 2034
- Growth Rate (2025-2034): 8.2% CAGR
- Largest Graft Versus Host Disease Market: United States
Key Factors Driving the Graft-Versus-Host Disease (GVHD) Market
Graft-Versus-Host Disease patient pool
In 2024, GvHD affected around 52K patients across the 7MM, with cases projected to reach 63K by 2034. This growth is driven by rising allogeneic transplants, better survival rates, and improved monitoring, expanding the GvHD market and demand for advanced GvHD treatments.
GvHD treatment approvals shaping the market
Recent approvals have significantly reshaped the GVHD treatment landscape. JAKAFI (Incyte) for steroid-refractory acute GVHD and multiple therapies for chronic GVHD, including IMBRUVICA (Pharmacyclics/AbbVie) and belumosudil (Kadmon), have expanded treatment options. In 2024, the GVHD market in the US was valued at approximately USD 965 million, reflecting a growing commercial opportunity.
GVHD pipeline and innovation focus
Ongoing GVHD clinical trials and translational programs continue to expand treatment options. Key pipeline therapies include CSL964 (CSL Behring), RGI-2001 (REGiMMUNE), EQ001 (Biocon Limited), MaaT013 (MaaT Pharma), and MEDI-507 (MedImmune LLC). Leading companies in the GVHD pipeline are Incyte, Kadmon, Pharmacyclics/AbbVie, Mesoblast, and others.
DelveInsight’s “Graft Versus Host Disease Market Insights, Epidemiology, and Market Forecast – 2034” report delivers an in-depth understanding of Graft Versus Host Disease, historical and forecasted epidemiology, as well as the Graft Versus Host Disease market trends in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
The Graft Versus Host Disease Treatment Market Report provides current treatment practices, emerging drugs, market share of individual therapies, and current and forecasted 7MM Graft Versus Host Disease market size from 2020 to 2034. The report also covers Graft Versus Host Disease treatment market practices/algorithms and unmet medical needs to curate the best opportunities and assess the market’s potential.
Graft versus Host Disease Understanding
Graft Versus Host Disease is an immune condition that occurs after transplant procedures when immune cells (T cells) from the donor (known as the graft or graft cells) attack the recipient patient host’s tissues (healthy cells); the disease is a side effect that is common after an allogeneic bone marrow transplant. In some cases, it can be even life-threatening. Before undergoing an allogeneic stem cell transplant, the patient will receive high doses of chemotherapy or radiation to destroy the diseased cells and prepare the body for the donor cells.
The two primary types of Graft Versus Host Disease are acute and chronic. An allogeneic transplant recipient may experience either form, either forms, or none at all. On average, Graft Versus Host Disease occurs in about 30–50% of patients who undergo allogeneic hematopoietic cell transplantation Graft Versus Host Disease diagnosis.
Graft Versus Host Disease diagnosis relies on clinical criteria, biopsies of affected organs (skin, liver, gastrointestinal tract), and exclusion of mimics like infection or drug reactions. While lab/imaging studies aid evaluation, a key challenge remains the lack of predictive biomarkers to preemptively identify at-risk patients or distinguish Graft Versus Host Disease from overlapping conditions. For Graft Versus Host Disease, diagnosis requires =1 distinctive manifestation (e.g., lichenoid lesions, scleroderma) after ruling out alternatives. Emerging blood-based biomarker panels may refine risk stratification and guide personalized immunosuppressive therapy, addressing current diagnostic limitations.
Graft Versus Host Disease Treatment
Graft Versus Host Disease is a major complication of allogeneic stem cell transplantation, with stem cells sourced from bone marrow, peripheral blood, or umbilical cord blood. Corticosteroids like prednisone and methylprednisolone are the primary first-line treatments, often combined with other immunosuppressants. Mild Graft Versus Host Disease is managed with topical steroids, while systemic cases require stronger immunosuppressive therapy. Graft Versus Host Disease is treated based on severity, with localized symptoms managed through topical treatments and more severe cases requiring systemic corticosteroids like prednisone.
Graft versus Host Disease Epidemiology
As the market is derived using a patient-based model, the Graft Versus Host Disease epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by total allogenic transplant cases, total Graft Versus Host Disease prevalence cases, type-specific cases of Graft Versus Host Disease, Graft Versus Host Disease cases by grading, Graft Versus Host Disease cases by organ involvement, Graft Versus Host Disease cases by grading, Graft Versus Host Disease cases by organ involvement, total treated cases of Graft Versus Host Disease, and mortality adjusted treated cases of Graft Versus Host Disease in the 7MM covering the United States, EU4 countries (Germany, France, Italy, and Spain) and the United Kingdom, and Japan from 2020 to 2034.
Key Findings from Graft Versus Host Disease Epidemiological Analyses and Forecast
- DelveInsight’s epidemiology model estimates that in 2023, there were approximately 57 thousand allogenic transplant cases and nearly 52 thousand cases of Graft Versus Host Disease across the 7MM which are expected to increase by 2034.
- In 2023, the US reported the highest number of Graft Versus Host Disease cases among the 7MM, with approximately 24 thousand, a figure expected to rise by 2034.
- In 2023, Germany reported the highest number of Graft Versus Host Disease cases among EU4 and the UK, with approximately 8 thousand cases, followed by Italy and France, with around 3 thousand cases each. Spain had the lowest number, with approximately 27 hundred cases.
- In 2023, Japan reported approximately 8 thousand Graft Versus Host Disease cases, a number projected to increase by 2034.
- In 2023, the US reported around 5 thousand cases of Graft Versus Host Disease and approximately 18 thousand prevalent cases of Graft Versus Host Disease over five years.
- In 2023, EU4 and the UK reported the following distribution of Graft Versus Host Disease cases by grading: around 2 thousand cases of Grade B[II] Graft Versus Host Disease, approximately 1 thousand cases of Grade C[III] Graft Versus Host Disease, and approximately 8 hundred cases of Grade D[IV] Graft Versus Host Disease.
- In 2023, Germany reported approximately 9 hundred Graft Versus Host Disease cases involving the skin, approximately 4 hundred cases involving the liver, and approximately 8 hundred cases involving the gastrointestinal tract.
- The UK reported approximately 6 hundred mild, approximately 1 thousand moderate, and approximately 8 hundred severe cases of Graft Versus Host Disease by grading in 2023.
- In 2023, Japan reported the following Graft Versus Host Disease cases: around 1 thousand cases involving the skin, approximately 3 thousand cases affecting the oral mucosa, approximately 2 thousand cases impacting the eyes, around 3 hundred cases involving the liver,approximately 8 hundred cases in the gastrointestinal tract, around 2 thousand cases affecting the lungs, approximately 3 hundred cases involving the genitals, and approximately 4 hundred cases related to the joints and fascia.
Graft Versus Host Disease Epidemiology Segmentation
- total allogenic transplant cases,
- total Graft Versus Host Disease prevalence cases,
- type-specific cases of Graft Versus Host Disease,
- Graft Versus Host Disease cases by grading,
- Graft Versus Host Disease cases by organ involvement,
- Graft Versus Host Disease cases by grading,
- Graft Versus Host Disease cases by organ involvement,
- total treated cases of Graft Versus Host Disease,
- mortality-adjusted treated cases of Graft Versus Host Disease
Graft versus Host Disease Recent Developments and Breakthroughs
- In May 2025, Mesoblast (ASX: MSB; Nasdaq: MESO) announced that the FDA has granted seven years of orphan drug exclusivity for Ryoncil® (remestemcel-L) to treat steroid-refractory acute graft-versus-host disease (SR-aGvHD) in pediatric patients aged 2 months and older.
- In March 2025, Signal12, Inc., a clinical-stage ophthalmic pharmaceutical company, announced alignment with the FDA on its Phase 3 clinical trial strategy for Pro-ocular™, a novel drop-free therapy aimed at revolutionizing the treatment of ocular Graft-versus-Host Disease (oGvHD).
- In December 2024, the FDA approved Mesoblast's cell therapy, Ryoncil, for treating graft-versus-host disease (Graft Versus Host Disease) following stem cell or bone marrow transplants. Ryoncil is the first mesenchymal stromal cell therapy approved for pediatric patients aged two months and older whose Graft Versus Host Disease symptoms have not responded to standard steroid therapy.
- In August 14, 2024, AstraZeneca’s IMFINZI (durvalumab) has received FDA approval for the treatment of adult patients with resectable early-stage (IIA-IIIB) non-small cell lung cancer (NSCLC) who do not have known epidermal growth factor receptor (EGFR) mutations or anaplastic lymphoma kinase (ALK) rearrangements. The regimen includes IMFINZI in combination with neoadjuvant chemotherapy before surgery and as adjuvant monotherapy after surgery.
Graft versus Host Disease Drugs Analysis
The drug chapter segment of the Graft Versus Host Disease clinical trials market report encloses a detailed analysis of Graft Versus Host Disease-marketed drugs and mid to late-stage (Phase III and Phase II) Graft Versus Host Disease pipeline drugs analysis. It also helps understand the Graft Versus Host Disease clinical trials details, expressive pharmacological action, agreements and collaborations, approval and patent details, advantages and disadvantages of each included drug, and the latest news and press releases.
Graft Versus Host Disease Marketed Drugs
-
JAKAFI MOA/JAKAVI (ruxolitinib): Incyte
JAKAFI MOA/JAKAVI (ruxolitinib) is a potent dual JAK1 and JAK2 inhibitor that exhibits low single-digit nanomolar biochemical IC50s for both kinases. The selectivity within the JAK family members is represented by a six-fold selectivity over TYK2 and approximately 130-fold selectivity over JAK3. Ruxolitinib demonstrates dose-dependent inhibition of JAK2/STAT signaling and inhibition of cell growth that is dependent on JAK2 activation.
-
IMBRUVICA (ibrutinib): Pharmacyclics (acquired by AbbVie)/ Janssen
IMBRUVICA (ibrutinib) is a first-in-class, oral, once-daily therapy that inhibits a protein called Bruton’s tyrosine kinase (BTK). BTK is a key signaling molecule in the B-cell receptor signaling complex that plays an important role in the survival and spread of malignant B cells and other serious, debilitating conditions. IMBRUVICA blocks signals that tell malignant B cells to multiply and spread uncontrollably.
Graft Versus Host Disease Emerging Drugs
-
ZEMAIRA (CSL 964, alpha-1 antitrypsin): CSL Behring
CSL 964 is an Alpha1-proteinase Inhibitor (A1-PI) being developed by CSL Behring to treat steroid-refractory a Graft Versus Host Disease and prevent a Graft Versus Host Disease in high-risk patients receiving an allogeneic HSCT. ZEMAIRA is approved to treat chronic augmentation and maintenance therapy in adults with alpha1-proteinase inhibitors. ZEMAIRA acts as augmentation therapy for increasing and maintaining serum levels and the levels of lung Epithelial Lining Fluid (ELF) of A1-PI.
The drug is currently in Phase III trials for treating steroid-refractory a Graft Versus Host Disease and in Phase II/III trials for preventing a Graft Versus Host Disease
-
EQ001 (itolizumab; Bmab600): Equillium/Biocon
EQ001 (Itolizumab; Bmab600) is a first-in-class immune-modulating antibody designed to inhibit CD6 to reduce the activation and trafficking of pathogenic T cells that release pro-inflammatory cytokines of autoimmune and inflammatory diseases like Graft Versus Host Disease, uncontrolled moderate-to-severe asthma, and lupus nephritis. Itolizumab targets the CD6-ALCAM signaling pathway to selectively downregulate pathogenic effector T cells (Teffs) while preserving Tregs critical for maintaining a balanced immune response.
Currently, EQ001 is being investigated in Phase III clinical study in combination with corticosteroids for the first-line treatment of Graft Versus Host Disease, which is in recruiting status. EQ001 topline data could see a potential acceleration to Q1 2025.
Graft Versus Host Disease Drugs Class Analysis
Graft Versus Host Disease treatment is evolving from broad immunosuppression to targeted and cell-based therapies. JAK inhibitors (e.g., ruxolitinib) are the backbone for steroid-refractory cases, while ROCK inhibitors (e.g., belumosudil) and anti-CSF-1R antibodies (e.g., axatilimab-csfr) address fibrosis in chronic Graft Versus Host Disease. MSC therapies (e.g., RYONCIL) and microbiome-based approaches (e.g., MaaT013) offer novel strategies for severe cases. IL-2Ra antagonists (e.g., basiliximab) and CD6 inhibitors (e.g., itolizumab) provide additional immunomodulation, though market adoption varies. Future treatments will likely focus on combination strategies targeting both inflammation and tissue repair.
Graft Versus Host Disease Market Outlook
Graft Versus Host Disease is a major complication of allogeneic stem cell transplants. Corticosteroids (e.g., prednisone) are the first-line treatment for a Graft Versus Host Disease, often combined with immunosuppressants like cyclosporine. Mild a Graft Versus Host Disease may require topical steroids, while severe cases need systemic corticosteroids. For Graft Versus Host Disease, prednisone is preferred for widespread symptoms, with localized treatments (e.g., steroid creams, eye drops) for mild, organ-specific cases.
- In August 2024, the US FDA approved NIKTIMVO (axatilimab-csfr) for treating Graft Versus Host Disease in adult and pediatric patients weighing at least 40 kg who have failed at least two prior lines of systemic therapy. Axatilimab-csfr is a monoclonal antibody targeting Colony-stimulating Factor-1 Receptors (CSF-1R) on monocytes and macrophages. By blocking CSF-1R, it reduces circulating proinflammatory and profibrotic monocytes and monocyte-derived macrophages, as evidenced by decreased nonclassical monocyte counts in nonclinical studies, while also inhibiting pathogenic macrophage activity in tissues. Syndax acquired exclusive global rights to develop and commercialize axatilimab from UCB in 2016.
- In September 2021, Syndax and Incyte entered into a global co-development and co-commercialization agreement for axatilimab in Graft Versus Host Disease and potential future indications.
- In 2017, the US FDA gave a green signal to IMBRVICA [Ibrutinib, AbbVie (Pharmacyclics)] for the treatment of adult patients suffering from Graft Versus Host Disease after the failure of one or more lines of systemic therapy. It was the first ever approved treatment targeting this specific patient pool. In September 2021, the PMDA approved IMBRUVICA to treat Graft Versus Host Disease after hematopoietic stem cell transplantation.
Graft Versus Host Disease Companies like CSL’s ZEMAIRA (CSL964, Alpha-1 antitrypsin), Equillium Bio’s EQ001 (Itolizumab), MaaT Pharma’s MaaT013, Medac’s MC0518, among others are involved in the development of drugs for the treatment of Graft Versus Host Disease.
Apart from this, several Graft Versus Host Disease drugs currently in the early stages of development include RLS-0071 by ReAlta Life Sciences, Vimseltinib by Deciphera Pharmaceuticals, ASC-930 by ASC Therapeutics, RGI-2001 by REGiMMUNE, CYP-001 by Cynata Therapeutics, arsenic trioxide (As2O3) by BioSenic (Medsenic), TRX103 (Tregs) by Tr1X, TCD601 (Siplizumab) by ITB-MED, F-652 by Evive Biotech, RHPRG4 by Lubris BioPharma, XBI302 by Xbiome, RG6287 by Genentech, ALTB-168 by AltruBio, and SER-155 by Seres Therapeutics.
- The total Graft Versus Host Disease Treatment Market Size in the 7MM was approximately USD 1,302 million in 2023 and is projected to increase during the forecast period (2025–2034).
- The Graft Versus Host Disease Treatment Market Size in the US was approximately USD 920 million in 2023 and is anticipated to increase due to the launch of emerging therapies.
- The total Graft Versus Host Disease Market Size of EU4 and the UK was calculated to be approximately USD 252 million in 2023, which was nearly 19% of the total market revenue for the 7MM.
- In 2023, Germany dominated the Graft Versus Host Disease Treatment Market among EU4 and the UK, generating around USD 98 million. France followed closely with approximately USD 41 million, while the UK recorded around USD 40 million.
- In 2023, the total Graft Versus Host Disease Treatment Market Size was approximately USD 130 million in Japan which is anticipated to increase during the forecast period (2025-2034).
- Estimates suggest that REZUROCK is expected to generate approximately USD 173 million by 2034 in the 7MM.
Graft versus Host Disease Drugs Uptake
This section focuses on the uptake rate of potential Graft Versus Host Disease drugs expected to be launched in the market during 2020–2034.
Further detailed analysis of emerging therapies drug uptake in the report…
Graft versus Host Disease Pipeline Development Activities
The Graft Versus Host Disease therapeutics market report provides insights into different Graft Versus Host Disease clinical trials within Phase III, Phase II, and Phase I. It also analyzes key Graft Versus Host Disease Companies involved in developing targeted therapeutics.
Latest KOL Views on Graft Versus Host Disease
To keep up with current market trends, we take KOLs and SMEs’ opinions working in the domain through primary research to fill the data gaps and validate our secondary research. Industry Experts contacted for insights on Graft Versus Host Disease evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake, along with challenges related to accessibility, including Medical/scientific writers, Medical Professionals, Professors, Directors, and Others.
DelveInsight’s analysts connected with 50+ KOLs to gather insights; however, interviews were conducted with 15+ KOLs in the 7MM. Centers like the University of Michigan, US, University of Minnesota, US, New York University, US, University Hospital Regensburg, Germany, Universite de Bordeaux, France, Hospital Saint-Antoine - Ap-Hp Sorbonne University, France, Università Cattolica del Sacro Cuore, Italy, Instituto de Investigaciones Biomédicas August Pi i Sunyer (IDIBAPS), Spain, University of Glasgow, the UK, University of Tokyo Hospital, Japan, and University Hospital Kyoto Prefectural University of Medicine, Japan, among others, were contacted. Their opinion helps understand and validate current and emerging therapy treatment patterns or Graft Versus Host Disease market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
Physician’s View
As per the KOLs from the US, genetic variations in cytokine-related genes, including TNF-a, IL-10, and IFN-?, contribute to the risk of Graft Versus Host Disease by influencing the intensity of the immune response, particularly the cytokine storm. While the associations between these polymorphisms and Graft Versus Host Disease remain variable and inconclusive, future advancements in donor selection are expected to incorporate both HLA and non-HLA genetic markers to refine transplant compatibility and improve patient outcomes.
As per the KOLs from France, acute Graft Versus Host Disease is a significant immune complication following alloHCT, leading to substantial morbidity and mortality. It results from donor immune cells attacking recipient tissues, particularly the skin, gastrointestinal tract, and liver. Diagnosis relies on clinical evaluation, with preventive therapies given universally but not always effective. Steroids remain first-line treatment, followed by the JAK2 inhibitor ruxolitinib.
As per the KOLs from Japan, for patients with acute Graft Versus Host Disease, early referral to a transplant center is crucial to access effective treatments and clinical trials, especially as the condition can become steroid-refractory. Graft Versus Host Disease typically begins with steroids, but referral to a specialized center ensures multidisciplinary care, addressing complications like steroid side effects, infections, and muscle weakness.
Graft Versus Host Disease Report Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the Analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving Graft Versus Host Disease treatment market landscape.
Conjoint Analysis analyzes multiple emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy. To analyze the effectiveness of these therapies, have calculated their attributed analysis by giving them scores based on their ability to improve atrial and ventricular dimension/function and ability to regulate heart rate.
Further, the therapies’ safety is evaluated wherein the adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials, which directly affects the safety of the molecule in the upcoming trials. It sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the route of administration, order of entry and designation, probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Graft Versus Host Disease Market Access and Reimbursement
JAKAFI MOA
IncyteCARES for JAKAFI MOA Savings Program
The IncyteCARES for JAKAFI MOA Savings Program offers eligible patients with commercial prescription insurance the opportunity to access JAKAFI MOA for as little as USD 0 per month. To qualify, individuals must have commercial prescription drug coverage, as those insured under federal or state government programs such as Medicare Part D, Medicare Advantage, Medicaid, or TRICARE are not eligible. Patients without prescription drug coverage also do not qualify. Additionally, eligibility requires residency in the US or Puerto Rico and a valid prescription for JAKAFI MOA for an FDA-approved indication.
Further details will be provided in the report.
The Graft Versus Host Disease therapeutics market report provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenarios, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Graft Versus Host Disease Market Report Scope
- The Graft Versus Host Disease clinical trials market report covers a segment of key events, an executive summary, and a descriptive overview, explaining its causes, signs and symptoms, pathogenesis, and currently available therapies.
- Comprehensive insight into the epidemiology segments and forecasts, the future growth potential of diagnosis rate, disease progression, and treatment guidelines have been provided.
- Additionally, an all-inclusive account of the current and emerging therapies and the elaborative profiles of late-stage and prominent therapies will impact the current treatment landscape.
- A detailed review of the Graft Versus Host Disease clinical trials market, historical and forecasted Graft Versus Host Disease treatment market size, Graft Versus Host Disease drugs market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The Graft Versus Host Disease clinical trials market report provides an edge while developing business strategies by understanding trends through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help shape and drive the 7MM Graft Versus Host Disease drugs market.
Graft versus Host Disease Clinical Trials Market Report Insights
- Patient-based Graft Versus Host Disease Market Forecasting
- Therapeutic Approaches
- Graft Versus Host Disease Pipeline Drugs Analysis
- Graft Versus Host Disease Market Size and Trends
- Existing and Future Graft Versus Host Disease Drugs Market Opportunity
Graft versus Host Disease Clinical Trials Market Report Key Strengths
- 10 years Graft Versus Host Disease Market Forecast
- The 7MM Coverage
- Graft Versus Host Disease Epidemiology Segmentation
- Key Cross Competition
- Attribute analysis
- Graft Versus Host Disease Drugs Uptake
- Key Graft Versus Host Disease Market Forecast Assumptions
Graft versus Host Disease Therapeutics Market Report Assessment
- Current Graft Versus Host Disease Treatment Market Practices
- Graft Versus Host Disease Unmet Needs
- Graft Versus Host Disease Pipeline Drugs Analyis Profiles
- Graft Versus Host Disease Drugs Market Attractiveness
- Qualitative Analysis (SWOT and Attribute Analysis)
- Graft Versus Host Disease Market Drivers
- Graft Versus Host Disease Market Barriers
Key Questions Answered in the Graft Versus Host Disease Market Report
Graft Versus Host Disease Clinical Trials Market Insights
- What was the total Graft Versus Host Disease therapeutics market, the Graft Versus Host Disease treatment market size by therapies, and Graft Versus Host Disease drugs market share (%) distribution in 2020, and what would it look like by 2034? What are the contributing factors for this growth?
- How will CSL964 affect the treatment paradigm of Graft Versus Host Disease?
- How will JAKAFI MOA compete with other upcoming products and marketed therapies?
- Which drug is going to be the largest contributor by 2034?
- What are the pricing variations among different geographies for approved and marketed therapies?
- How would future opportunities affect the market dynamics and subsequent analysis of the associated trends?
Graft Versus Host Disease Epidemiology Insights
- What are the disease risks, burdens, and Graft Versus Host Disease unmet needs? What will be the growth opportunities across the 7MM with respect to the patient population pertaining to Graft Versus Host Disease?
- What is the historical and forecasted Graft Versus Host Disease patient pool in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan?
- Out of the countries mentioned above, which country would have the highest diagnosed prevalent Graft Versus Host Disease population during the forecast period (2025–2034)?
- What factors are contributing to the growth of Graft Versus Host Disease cases?
Current Graft Versus Host Disease Treatment Market Scenario, Marketed Drugs, and Emerging Therapies
- What are the current options for the Graft Versus Host Disease treatment? What are the current clinical and treatment guidelines for treating Graft Versus Host Disease?
- How many Graft Versus Host Disease Companies are developing therapies for the Graft Versus Host Disease treatment?
- How many emerging therapies are in the mid-stage and late stage of development for treating Graft Versus Host Disease?
- What are the recent novel therapies, targets, mechanisms of action, and technologies developed to overcome the limitations of existing therapies?
- What is the cost burden of current treatment on the patient?
- Patient acceptability in terms of preferred treatment options as per real-world scenarios?
- What are the accessibility issues of approved therapy in the US?
- What is the 7MM historical and forecasted Graft Versus Host Disease Drugs Market?
Reasons to Buy the Graft Versus Host Disease Report
- The Graft Versus Host Disease clinical trials market report will help develop business strategies by understanding the latest trends and changing treatment dynamics driving the Graft Versus Host Disease drugs market.
- Insights on patient burden/disease Graft Versus Host Disease Prevalence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years.
- Understand the existing Graft Versus Host Disease drugs market opportunities in varying geographies and the growth potential over the coming years.
- The distribution of historical and current patient share is based on real-world prescription data in the US, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
- Identifying upcoming solid players in the Graft Versus Host Disease drugs market will help devise strategies to help get ahead of competitors.
- Detailed analysis and ranking of class-wise potential current and emerging therapies under the conjoint analysis section to provide visibility around leading classes.
- Highlights of Access and Reimbursement policies for Graft Versus Host Disease, barriers to accessibility of approved therapy, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing Graft Versus Host Disease drugs market so that the upcoming players can strengthen their development and launch strategy.
Dive deeper into our insightful blogs, engaging videos, and captivating infographics to expand your knowledge:-

.jpg)