Intratumoral Cancer Therapies Market Summary
- The Intratumoral Cancer Therapies market in the 7MM was valued at approximately USD 115 million in 2025.
- The Intratumoral Cancer Therapies market is projected to grow at a CAGR of 49.10%, reaching USD 4,193 million by 2034 in leading countries (US, EU4, UK and Japan).
Intratumoral Cancer Therapies Market and Epidemiology Analysis
- Intratumoral Cancer Therapies Treatments are delivered directly into tumors, targeting cancer cells locally, often with minimal impact on surrounding healthy tissue.
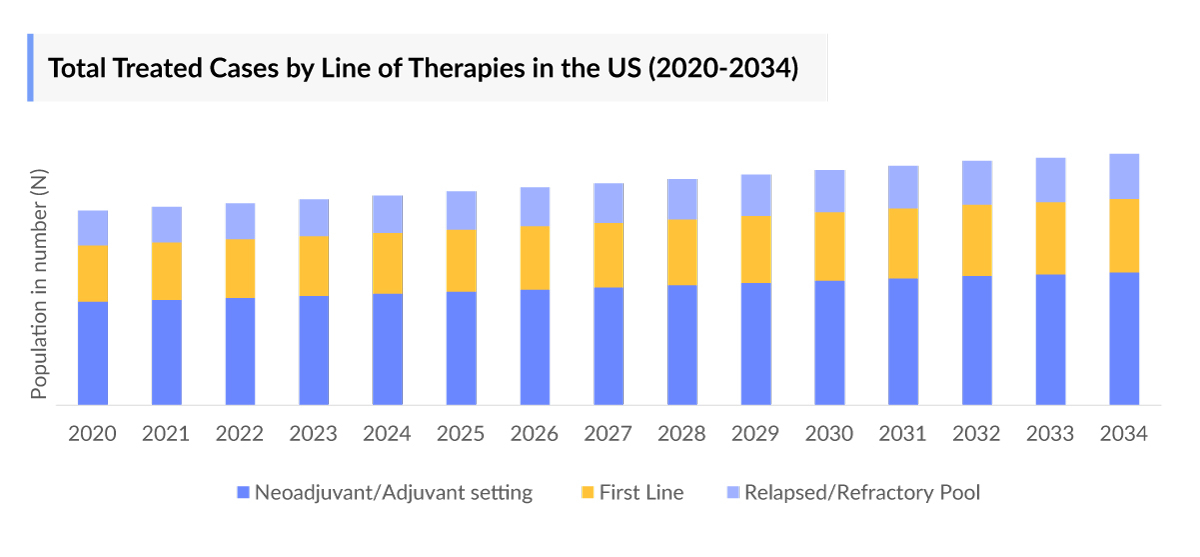
- Among the selected indications for intratumoral therapies, non-melanoma accounted for the highest number of cases across the 7MM and is anticipated to increase at ~ 2.0% in the coming decade. Further, in the US, breast cancer constitutes approximately 30% of cancer cases, with the Intratumoral Cancer Therapies Incidence rate steadily increasing.
- Currently, only IMLYGIC is approved by the US FDA for melanoma treatment and garnered more than 90% of the total intratumoral therapies market.
- Various intratumoral therapy approaches, including immune-potentiating cytokines, TLR and STING agonists, T-cell checkpoint inhibitors, suppressive cytokine inhibitors or traps, OVs, and plasmid DNA, are in clinical development for melanoma patients.
- Among the emerging Intratumoral Cancer Therapies, OncoSec Medical’s TAVO (tavokinogene telseplasmid), a plasmid-based Interleukin-12 is expected to generate the highest revenue.
- The rapid advancement of Intratumoral Cancer Therapies and active involvement of big pharmaceutical Intratumoral Cancer Therapies Companies like Nanobiotix, Replimune, Sirnaomics, and others, suggests a promising future, poised to revolutionize the cancer treatment landscape and intratumoral cancer therapies market.
- In March 2024, ImmVira announced that the US Food and Drug Administration (FDA) has granted Fast Track designation for the oncolytic virus product MVR-T3011 IT (intratumoral injection) for the treatment of recurrent head and neck squamous cell cancer with disease progression after platinum-based chemotherapy and at least one prior line of anti-PD1/PDL1 therapy.
- In November 2023, Genelux announced that the US FDA has granted Fast Track designation for the development program of Olvi-Vec (olvimulogene nanivacirepvec) for the treatment of patients with platinum resistant/refractory ovarian cancer.
Intratumoral Cancer Therapies Market size and forecast
- 2025 Intratumoral Cancer Therapies Market Size: USD 115 million
- 2034 Projected Intratumoral Cancer Therapies Market Size: USD 4,193 million
- Intratumoral Cancer Therapies Growth Rate (2025-2034): 49.10% CAGR
- Largest Intratumoral Cancer Therapies Market: United States
Request for Sample Page of the "Intratumoral Cancer Therapies Treatment Market"
Key Factors Driving Intratumoral Cancer therapies Market
Rising Incidence of Cancers Eligible for Intratumoral Therapies
Intratumoral therapies deliver treatments directly into tumors, targeting cancer cells locally while minimizing effects on surrounding tissue. Across the 7MM, non-melanoma cancers accounted for the highest number of cases and are projected to grow at ~2.0% annually over the coming decade. This rising incidence highlights the expanding patient pool and demand for novel, localized cancer treatments.
Current Landscape of Intratumoral Cancer Therapies
At present, IMLYGIC remains the only FDA-approved intratumoral therapy for melanoma and has captured more than 90% of the current market. Despite this, the field is rapidly evolving, with therapies targeting diverse mechanisms including immune-potentiating cytokines, TLR and STING agonists, T-cell checkpoint inhibitors, suppressive cytokine traps, oncolytic viruses (OVs), and plasmid DNA-based therapies. Among these, OncoSec Medical’s TAVO (tavokinogene telseplasmid, an IL-12 plasmid therapy) is expected to generate notable revenue as a leading emerging therapy.
Recent Regulatory Milestones in Intratumoral Therapies
The pace of development is accelerating, supported by FDA designations for innovative candidates. In March 2024, ImmVira received Fast Track designation for MVR-T3011 IT, an oncolytic virus therapy for recurrent head and neck squamous cell cancer. Similarly, in November 2023, Genelux secured Fast Track designation for Olvi-Vec (olvimulogene nanivacirepvec) in platinum-resistant/refractory ovarian cancer. These milestones emphasize growing regulatory support for intratumoral treatments.
Strong Clinical Trial Pipeline in Intratumoral Cancer Therapies
A robust pipeline is underway, with key investigational therapies including Vidutolimod + nivolumab (Regeneron Pharmaceuticals), LTX-315 (Lytix Biopharma), AIV001 (AiViva BioPharma), and others. These candidates reflect the diverse strategies being employed to enhance tumor-specific immune responses and improve patient outcomes. Leading players include Nanobiotix, Replimune, Sirnaomics, OncoSec Medical, Daiichi Sankyo, Amgen, Merck & Co (Viralytics), Moderna Therapeutics, Idera Pharmaceuticals, Philogen, Lokon Pharma, Hookipa Biotech, Vyriad, Intensity Therapeutics, and many others.
DelveInsight’s “Intratumoral Cancer Therapies Treatment Market Insight, Epidemiology and Market Forecast – 2034” report delivers an in-depth understanding of the Intratumoral cancer therapies, historical and Competitive Landscape as well as the Intratumoral cancer therapies market trends in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
The Intratumoral Cancer Therapies Treatment Market Report provides current treatment practices, emerging drugs, market share of individual therapies, and current and forecasted 7MM Intratumoral cancer therapies market size from 2020 to 2034. The report also covers current Intratumoral cancer therapies treatment market practices/algorithms and Intratumoral cancer therapies unmet needs to curate the best opportunities and assess the market’s potential.
|
Study Period |
2020 to 2034 |
|
Forecast Period |
2023-2034 |
|
Geographies Covered |
|
|
Intratumoral Cancer Therapies Treatment Market |
|
|
Intratumoral Cancer Therapies Market Size | |
|
Intratumoral Cancer Therapies Companies |
|
Intratumoral Cancer Therapies Understanding
Intratumoral Cancer Therapies Overview
Cancer is a broad term for diseases caused by abnormal cells quickly dividing and spreading to other tissues and organs. It is one of the world’s major causes of death. Intratumoral therapy is a promising treatment option for several cancer types. Intratumoral immunotherapy is a revolutionary cancer treatment that uses sophisticated antibodies directly injected into the cancer tumors instead of through a vein (intravenously).
These therapies aim to deliver immunostimulatory products directly into a tumor lesion (primary or metastatic) to prime and/or boost an antitumor immune response. Intratumoral therapy aims to generate a systemic immune response against the tumor upon local immune stimulation due to the circulation in the blood and lymph of immune cells and antibodies. Therefore, the objective of such a therapeutic strategy is to use the tumor as its vaccine and generate a polyclonal adaptive immune response mediated by T and/or B cells against pre-existing tumor-specific antigens (TSA) or tumor-associated antigens (TAA).
Further details related to country-based variations are provided in the report
Intratumoral Cancer Therapies Treatment
The Intratumoral Cancer Therapies treatment landscape has rapidly evolved, with several promising advancements in recent years. Due to the increasing interest in new Intratumoral cancer therapies, companies like Nanobiotix, Replimune, and Sirnaomics have redirected their efforts toward advancing their Intratumoral cancer therapies clinical trials development program. This program is currently in the lead optimization stage. The development of Intratumoral cancer therapies has the potential to address a significant unmet need among patients with various types of tumors.
The first successful intratumoral immunotherapy was observed at the end of the 19th century following intratumoral injections of pro-inflammatory bacterial extracts. Previously, the interest of researchers was not too much in Intratumoral therapies. In 2015, with the approval of T-Vec, an oncolytic virus to treat melanoma, the interest increased, and several companies are developing intratumoral drugs. Intratumoral therapy has several advantages over the existing treatment, e.g., intratumoral therapy causes fewer side effects. Preclinical studies have estimated that intratumoral therapy has improved patients’ outcomes suffering from various cancer diseases
Intratumoral Cancer Therapies Drugs Analysis
The drug chapter segment of the Intratumoral cancer therapies market outlook reports encloses a detailed analysis of Intratumoral cancer therapies marketed drugs and late-stage (Phase III and Phase I/II) Intratumoral cancer therapies pipeline drugs. It also helps understand the Intratumoral cancer therapies clinical trials details, expressive pharmacological action, agreements and collaborations, approval and patent details, advantages and disadvantages of each included drug, and the latest Intratumoral cancer therapies news and press releases.
Intratumoral cancer therapies Marketed Drugs
-
IMLYGIC (talimogene laherparepvec): Amgen
IMLYGIC (T-VEC) by Amgen, the first oncolytic virus, was approved by the US FDA for the local treatment of unresectable cutaneous, subcutaneous, and nodal lesions in patients with melanoma recurrent after the initial surgery in 2015. After few months, in December 2015, the European Commission (EC) also approved it for the treatment of adults with unresectable melanoma that is regionally or distantly metastatic (Stage IIIB, IIIC, and IVM1a), with no bone, brain, lung or other visceral diseases. It is a genetically modified herpes simplex virus type 1 designed to replicate within tumors and produce an immunostimulatory protein called granulocyte-macrophage colony-stimulating factor (GM-CSF).
-
DELYTACT (G47Δ): Daiichi Sankyo
DELYTACT is a triple-mutated, replication-conditional herpes simplex virus type 1 (HSV-1) that has been developed to replicate only in cancer cells. In 2021, it received conditional and time-limited approval from the Japan Ministry of Health, Labour and Welfare (MHLW) to commercialize only at hospitals (served as trial sites) for the treatment of patients with malignant glioma. for a duration of 7 years. Approval was based on data from a single-arm phase II trial which showed that DELYTACT improved chances of survival at one year in patients with residual or recurrent glioblastoma previously treated with radiotherapy and temozolomide chemotherapy who residual or recurrent disease. It is the first oncolytic virus therapy to be launched anywhere in the world for brain cancer, and only the third oncolytic virus therapy to be made available to patients worldwide.
Note: Detailed current therapies assessment will be provided in the full report of Intratumoral cancer therapies
|
Approved drug |
Company |
Condition |
Molecule type |
Intratumoral Cancer Therapies MOA |
RoA |
|
DELYTACT (teserpaturev/G47∆/ DS-1647) |
Daiichi Sankyo |
Malignant glioma |
Oncolytic Virus |
Cell death stimulants |
Intratumoral |
|
IMLYGIC (Talimogene laherparepvec) |
Amgen |
Local treatment of unresectable cutaneous, subcutaneous, and nodal lesions in patients with melanoma recurrent after initial surgery |
Oncolytic virus |
Replicates inside tumor cells and production of GM-CSF |
Intratumoral |
Intratumoral Cancer Therapies Emerging Drugs
-
HENSIFY: Nanobiotix
HENSIFY, a first-in-class product, introduces a new, physical Intratumoral Cancer Therapies mechanism of action. It was designed by Nanobiotix to physically destroy tumors and activate the immune system for local control and systemic disease treatment when combined with radiation therapy. In addition to HENSIFY, NBTXR3 is currently under evaluation in various other indications such as lung, head, neck, liver, and prostate cancer. It is an aqueous suspension of crystalline hafnium oxide (HfO2) nanoparticles designed for injection directly into a tumor before a patient’s first standard radiotherapy treatment. When exposed to ionizing radiation, HENSIFY amplifies the localized, intratumor killing effect of that radiation. X-ray dose delivered to the tumor is magnified, while the dose passing through healthy tissues remains unchanged. In February 2020, Nanobiotix announced that the US FDA had granted FTD for the investigation of NBTXR3 activated by radiation therapy, with or without cetuximab, for treating patients with locally advanced head and neck squamous cell cancer who are not eligible for platinum-based chemotherapy.
Currently, it is in Phase III of Intratumoral Cancer Therapies Clinical Trials development for the treatment of locally advanced head and neck squamous cell carcinoma.
-
STP705 (cotsiranib): Sirnaomics
Sirnaomics’ leading product candidate, STP705, is a siRNA (small interfering RNA) therapeutic that takes advantage of a dual-targeted inhibitory property and polypeptide nanoparticle (PNP)-enhanced delivery to knock down both TGF-β1 and COX-2 gene expression directly. The drug is composed of two siRNA oligonucleotides targeting TGF-β1 and COX-2 mRNA, respectively, and formulated in nanoparticles with a proprietary histidine-lysine co-polymer (HKP) peptide. There are currently three product pipeline programs prioritized by STP705: a late-stage clinical development for Squamous Cell Carcinoma in situ (isSCC), completion of Phase II for Basal Cell Carcinoma (BCC), and Phase I for the fat remodeling. For other indications, STP705 has received an Orphan Drug Designation for the treatment of cholangiocarcinoma (CCA) and primary sclerosing cholangitis (PSC).
Note: Detailed emerging therapies assessment will be provided in the final report.
|
Vidutolimod + nivolumab |
Regeneron Pharmaceuticals |
TLR-9 receptor agonist |
II/III
|
NCT04695977 | ||
|
LTX-315 |
Lytix Biopharma |
Oncolytic Peptides |
II |
NCT04796194 | ||
|
AIV001 |
AiViva BioPharma |
Superficial Basal Cell Carcinoma; Nodular Basal Cell Carcinoma |
Multi-kinase inhibitor |
I/II |
NCT04470726 | |
Intratumoral Cancer Therapies Market Outlook
The Intratumoral cancer therapies market outlook is expected to grow significantly in the coming years. This is due to the increasing number of patients who are being diagnosed with cancer, the growing awareness regarding these therapies, and the increasing number of intratumoral cancer therapies that are under investigation. To date, approved intratumoral cancer therapies include IMLYGIC (talimogene laherparepvec), and DELYTACT (G47Δ)(only approved in Japan). Currently, IMLYGIC is dominating the intratumoral cancer therapies market, however vidutolimod, and RP1 is likely to have a substantial impact on the intratumoral market landscape in the coming decade.
Several Intratumoral cancer therapies Companies, including Nanobiotix, Sirnaomics, Replimune, and others, are involved in developing intratumoral cancer therapies for various indications. At present, melanoma garnered the highest revenue among the selected cancer indications. In the upcoming years, the intratumoral cancer therapies are likely to be considered as the promising therapy for non-melanoma skin cancer.
- The United States accounts for approximately 80% Intratumoral Cancer Therapies Market Share.
- Among EU4 and the UK, Germany accounted for the highest Intratumoral Cancer Therapies Market Share, followed by France whereas Spain accounted for the lowest market.
Overall, this is an exciting new treatment with great potential for development. The maturation of current studies over the next few years will lead to a better understanding of Intratumoral Cancer Therapies and define their role in the therapy of cancer.
Intratumoral Cancer Therapies Drugs Uptake
This section focuses on the uptake rate of potential approved and emerging intratumoral cancer therapies expected to be launched in the market during 2020–2034.
Intratumoral Cancer Therapies Pipeline Development Activities
The Intratumoral cancer therapies pipeline segment provides insights into different therapeutic candidates in Phase III, Phase II, and Phase I stage. It also analyzes Intratumoral cancer therapies Companies involved in developing targeted therapeutics. The presence of numerous Intratumoral cancer therapies drugs under different stages is expected to generate immense opportunity for intratumoral cancer therapies drugs market growth over the forecast period.
Pipeline Development Activities
The Intratumoral cancer therapies pipeline segment covers information on collaborations, acquisitions and mergers, licensing, and patent details for Intratumoral Cancer emerging therapies. The increasing strategic collaborations among major market players to enhance the growth of their Intratumoral Cancer Therapies pipeline products are anticipated to drive market expansion
Latest KOL Views on Intratumoral Cancer Therapies
To keep up with current and future Intratumoral Cancer Therapies Drugs Market Trends, we take Industry Experts’ opinions working in the domain through primary research to fill the data gaps and validate our secondary research. Industry experts were contacted for insights on evolving Intratumoral Cancer Therapies Treatment Market Landscape, patient reliance on conventional therapies, patient therapy switching acceptability, drug uptake, along with challenges related to accessibility.
DelveInsight’s analysts connected with 30+ KOLs to gather insights; however, interviews were conducted with 15+ KOLs in the 7MM. Their opinion helps understand and validate current and emerging therapy treatment patterns or Intratumoral Cancer Therapies market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the Intratumoral cancer therapies unmet needs.
Intratumoral Cancer Therapies Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving Intratumoral cancer therapies treatment landscape.
Conjoint Analysis analyzes multiple approved and emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy. In efficacy, the trial’s primary and secondary outcome measures are evaluated; for instance, in event-free survival, one of the most important primary outcome measures is event-free survival and overall survival.
Further, the therapies’ safety is evaluated wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Intratumoral Cancer Therapies Treatment Market Access and Reimbursement
Health technology assessments (HTAs) evaluate published literature according to evidence-based criteria to present a high-quality scientific synthesis of available evidence regarding clinical benefits, harms, economic consequences, and ethical or social issues. HTAs are increasingly leveraged as an essential requirement for healthcare decision-making. Ideally, an HTA is expected to evaluate the long-term benefits and risks of a given medical intervention to its costs. Several initiatives have already emerged in the past.
In the UK, IMLYGIC was accepted for use with the manufacturer agreeing to apply a discount to the list price under a patient access scheme (PAS); however, in Germany, IMLYGIC was assessed to have no added benefit due to the selection of inappropriate comparators. The report further provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of approved therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Intratumoral Cancer Therapies Treatment Market Report Scope
- The Intratumoral Cancer Therapies treatment market report covers a segment of key events, an executive summary, and a descriptive overview.
- Comprehensive insight into the Competitive Landscape, and forecasts, the future growth potential of diagnosis rate, disease progression, and treatment guidelines have been provided.
- Additionally, an all-inclusive account of the current and emerging therapies and the elaborative profiles of late-stage and prominent therapies will impact the current Intratumoral Cancer Therapies treatment landscape.
- A detailed review of the Intratumoral Cancer Therapies treatment market, historical and forecasted Intratumoral Cancer Therapies treatment market size, Intratumoral Cancer Therapies market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The patient-based Intratumoral Cancer Therapies market forecasting report provides an edge while developing business strategies, by understanding trends, through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help shape and drive the 7MM Intratumoral Cancer Therapies Drugs Market.
Intratumoral Cancer Therapies Therapeutics Market Report Insights
- Intratumoral Cancer Therapies Targeted Patient Pool
- Therapeutic Approaches
- Intratumoral Cancer Therapies Pipeline Analysis
- Intratumoral Cancer Therapies Treatment Market Size
- Intratumoral Cancer Therapies Market Trends
- Existing and future Intratumoral Cancer Therapies Therapeutics Market Opportunity
Intratumoral Cancer Therapies Therapeutics Market Report Key Strengths
- 10 Years Intratumoral Cancer Therapies Market Forecast
- The 7MM Coverage
- Key Cross Competition
- Intratumoral Cancer Therapies Drugs Uptake
- Key Intratumoral Cancer Therapies Market Forecast Assumptions
Intratumoral Cancer Therapies Treatment Market Report Assessment
- Current Intratumoral Cancer Therapies Treatment Market Practices
- Intratumoral Cancer Therapies Unmet Needs
- Intratumoral Cancer Therapies Pipeline Product Profiles
- Intratumoral Cancer Therapies Therapeutics Market Attractiveness
- Qualitative Analysis (SWOT)
Key Questions Answered in the Intratumoral Cancer Therapies Market Report
- What was the Intratumoral Cancer Therapies Treatment market size, the Intratumoral Cancer Therapies market size by therapies, Intratumoral Cancer Therapies market share (%) distribution in 2023, and what would it look like in 2034? What are the contributing factors for this growth?
- Which drug is going to be the largest contributor in 2034?
- Which is the most lucrative Intratumoral Cancer Therapies Drugs Market?
- Which drug type segment account for maximum Intratumoral Cancer Therapies sales?
- What are the pricing variations among different geographies for approved therapies?
- How has the reimbursement landscape for Intratumoral Cancer Therapies evolved since the first one was approved? Do patients have any access issues that are driven by reimbursement decisions?
- What are the disease risk, burdens, and Intratumoral Cancer Therapies unmet needs? What will be the growth opportunities across the 7MM with respect to the patient population pertaining to Intratumoral Cancer Therapies?
- What are the key factors hampering the Intratumoral Cancer Therapies Treatment Market growth?
- What are the recent novel therapies, targets, Intratumoral Cancer Therapies mechanisms of action, and technologies developed to overcome the limitation of existing therapies?
- What key designations have been granted for the Intratumoral Cancer Therapies Emerging Therapies?
- What is the cost burden of approved therapies on the patient?
- Patient acceptability in terms of preferred treatment options as per real-world scenarios?
- What are the country-specific accessibility issues of expensive, recently approved therapies?
Reasons to buy Intratumoral Cancer Therapies Market Report
- The Intratumoral Cancer Therapies Therapeutics Market report will help develop business strategies by understanding the latest trends and changing treatment dynamics driving the Intratumoral Cancer Therapies Drugs Market.
- Understand the existing Intratumoral Cancer Therapies Drugs Market opportunity in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
- Identifying strong upcoming Intratumoral Cancer Therapies companies in the market will help devise strategies to help get ahead of competitors.
- Detailed analysis and ranking of class-wise potential current and emerging therapies under the conjoint analysis section to provide visibility around leading classes.
- Highlights of Access and Reimbursement policies of approved therapies, barriers to accessibility of expensive off-label therapies, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet need of the existing Intratumoral Cancer Therapies Drugs Market so that the upcoming players can strengthen their development and launch strategy.
Access Exclusive Data Now! Click here to Read More about the Related Articles

.jpg)

.jpg)