Intratumoral Cancer Therapies Market Summary
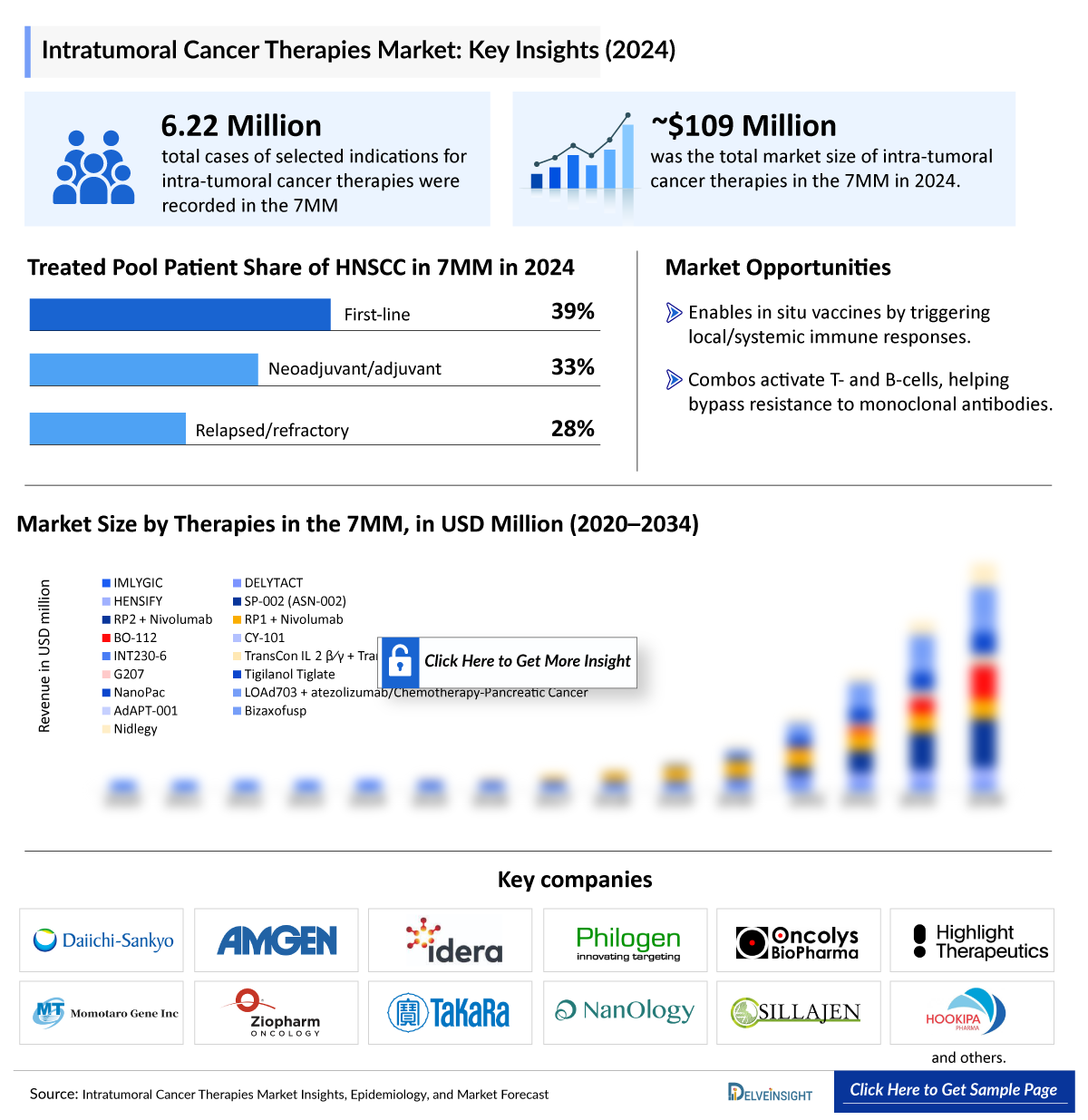
- The Intra-Tumoral Cancer Therapies market in the 7MM is projected to grow at a significant CAGR by 2034 from ~109 Million in 2024 in leading countries (US, EU4, UK and Japan).
Intra-Tumoral Cancer Therapies Market and Epidemiology Analysis
- Intra-tumoral cancer therapies refer to any therapy delivered in very close anatomical proximity to a tumor with the intention of direct uptake by tumors or tumor cells.
- Intra-tumoral cancer therapies deliver antitumor agents directly into tumors to induce local tumor death, release antigens, and activate a localized immune response—potentially triggering systemic effects (abscopal effect). It also reduces systemic toxicity while achieving high drug levels at the tumor site, offering an advantage for patients unsuitable for systemic treatments.
- Multiple intra-tumoral cancer therapies approaches, including radioenhancer (nanoparticle), recombinant fusion proteins, oncolytic therapy, gene therapy, synthetic dsRNA complex, and others, etc., are under clinical development for melanoma patients.
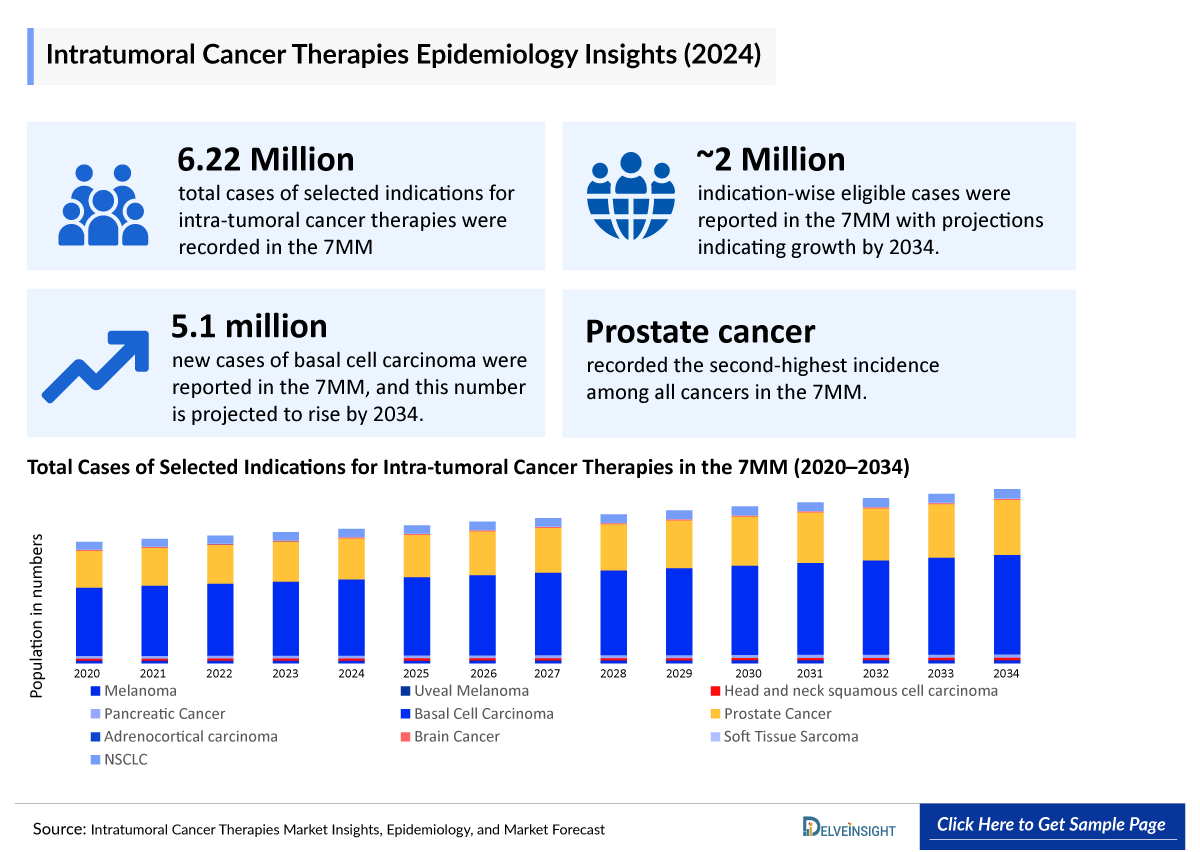
- In 2024, the total cases of selected indications for ITCT were 8,965,500 cases in the 7MM, which is anticipated to increase by 2034.
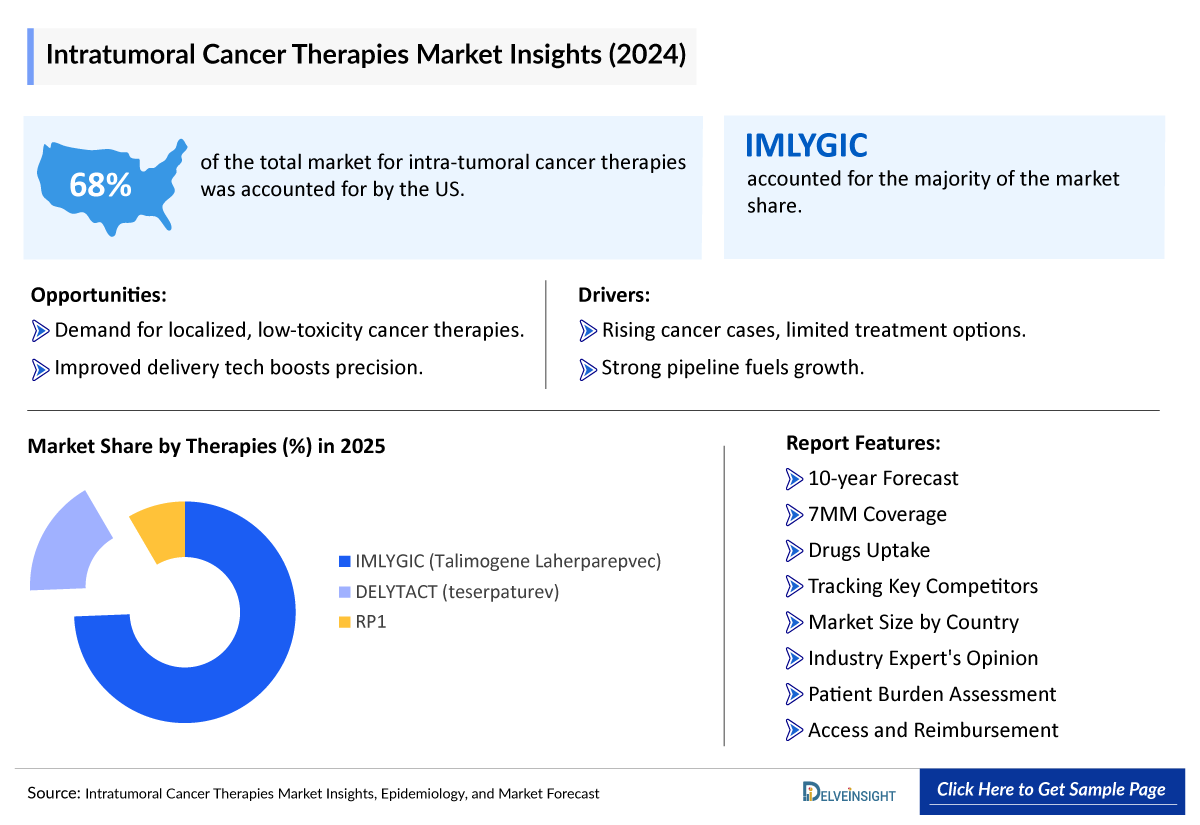
- The US accounts highest market size of intra-tumoral cancer therapies, owing to the highest number of intra-tumoral cancer therapies-based disease cases, higher treatment costs, and the launch of emerging drugs during the forecast period, followed by Germany and Italy by 2034.
- Currently, only three intra-tumoral cancer therapies are approved; namely, IMLYGIC (Talimogene laherparepvec/T-VEC; Amgen), approved in the US and Europe in 2015, DELYTACT (teserpaturev/G47∆; Daiichi Sankyo) approved in Japan in 2021, and HENSIFY in Europe in 2019.
- As per the JP Morgan Healthcare Conference presentation 2025, RP1 is positioned to have an independent commercial launch in the second-half of 2025.
- In March 2025, NANOBIOTIX announced topline data from the completed dose-escalation portion of a Phase I study, conducted under the sponsorship of The University of Texas MD Anderson Cancer Center, evaluating NBTXR3 (JNJ-1900) activated by radiation therapy as a second-line or later (2L+) treatment for patients with locally advanced NSCLC eligible for re-irradiation.
- In the 7MM, in 2024, among all the indications, Basal Cell Carcinoma (BCC) accounted for the highest number of diagnosed prevalent cases, while Adrenocortical Carcinoma (ACC) occupied the bottom of the ladder.
- A diverse group of biopharmaceutical companies, including Amgen, Candel Therapeutics, Nanobiotix, Daiichi Sankyo, Replimune, Lytix Biopharma, Highlight Therapeutics, and others, are actively engaged in the development of intra-tumoral cancer therapies across various indications, reflecting a growing strategic focus on localized oncology treatments and an increasingly competitive pipeline.
- By 2034, CAN-2409 under development for prostate cancer, NSCLC, and pancreatic cancer is expected to achieve the highest market size across the US, EU4 and the UK, and Japan.
- In January 2025, Stamford Pharmaceuticals announced positive results for its ASN-002-003 Phase IIa trial evaluating SP-002 in combination with vismodegib in subjects presenting with multiple BCC.
- In January 2025, Lokon Pharma AB announced that the FDA has granted Fast Track Designation (FTD) for the company product candidate LOAd703 for the treatment of pancreatic cancer.
- As per Cytovation ASA, the company is anticipating initiating Phase II of CY-101 as monotherapy for ACC in the first-half of 2025 and as a combination with KEYTRUDA in Phase I/II for CRC in the second-half of 2025.
Request for Unlocking the Sample Page of the "Intratumoral Cancer Therapies Treatment Market"
DeveInsight’s “Intra-Tumoral Cancer Therapies Treatment Market Insights, Epidemiology, and Market Forecast – 2034” report delivers an in-depth understanding of Intra-Tumoral Cancer Therapies, historical and forecasted epidemiology as well as Intra-Tumoral Cancer Therapies market trends in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
The Intra-Tumoral Cancer Therapies Treatment Market Report provides current treatment practices, emerging drugs, market share of individual therapies, and current and forecasted the 7MM Intra-Tumoral Cancer Therapies market size from 2020 to 2034. The report also covers current Intra-Tumoral Cancer Therapies treatment practices/algorithms and unmet medical needs to curate the best opportunities and assess the market’s potential.
Intra-Tumoral Cancer Therapies Treatment Market
Cancer is a diverse disease as the signs, symptoms, and treatment vary among the various cancer types, making it essential to develop a specified therapy for the patients. Currently available cancer treatment options include immunotherapy, radiotherapy, chemotherapy, targeted drug therapy, and others. Although these therapies have improved the OS and outcome of the patient, they possess some limitations and cannot benefit all Intra-Tumoral Cancer Therapies Patients. Scientists are focused on developing treatment options that can overcome the challenges, and one such emerging treatment option is Intra-Tumoral Cancer Therapies.
Intra-tumoral immunotherapy is a revolutionary cancer treatment that uses sophisticated antibodies directly injected into the cancer tumors instead of through a vein (intravenously). These therapies aim to deliver immunostimulatory products directly into a tumor lesion (primary or metastatic) to prime and/or boost an antitumor immune response.
Mechanism of Intra-Tumoral Cancer Therapies
The unchecked proliferation of the cells is the characteristic feature of tumor tissue. The immune system usually recognizes and eliminates nascent tumors; however, in immunosuppressive tumors, the malignant cells proliferate and are generally undetected by the immune system. Regulatory T cells (Tregs) are attracted to the tumor by chemokines and aid in suppressing Antigen-presenting Cells (APCs) that may otherwise stimulate a response against tumor antigens. Additionally, tumor cells can secrete anti-inflammatory and regulatory cytokines (i.e., TGFß, IL-10) that facilitate cancer growth and directly prevent DC activation.
Alternatively, immunostimulants can be used to overcome the suppressive environment within the tumor, which works by recruiting immune cells or by activating the immune system to recognize and attack cancer cells. Immunostimulants can activate immune cells in the presence of tumor antigen, traffic to lymph nodes, and then activate tumor antigen-specific T cells via cross-presentation. Antigen-specific T cells may circulate back to the tumor or distal tumors, instigating tumor-cell killing.
Intra-Tumoral Cancer Therapies Epidemiology
The Intra-Tumoral Cancer Therapies epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by Total cases of selected indications in the 7MM, Indication wise target patient pool of Intra-Tumoral Cancer Therapies in the 7MM, and Indication wise treated cases of Intra-Tumoral Cancer Therapies in the 7MM covering the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan from 2020 to 2034.
- In 2024, the total the indication-wise eligible cases were 19,39,000 in the 7MM, which is anticipated to increase by 2034.
- In Japan, NSCLC accounted for highest number of indication-wise eligible cases, i.e., ~67,000 cases, in 2020. These cases are expected to increase during the forecast period (2025–2034).
- In 2024, the total cases of selected indications for Intra-Tumoral Cancer Therapies were approximately 62,20,000 in the 7MM, which is anticipated to increase by 2034.
Intra-Tumoral Cancer Therapies Drugs Market Chapters
The drug chapter segment of the Intra-Tumoral Cancer Therapies Therapeutics Market Report encloses a detailed analysis of the marketed and late-stage (Phase III, and II) Intra-Tumoral Cancer Therapies Pipeline Drugs analysis. The Intra-Tumoral Cancer Therapies marketed drugs segment encloses HENSIFY (NBTXR3) (NANOBIOTIX/Janssen Pharmaceutical), DELYTACT (teserpaturev) (Daiichi Sankyo) etc., Furthermore, the current Intra-Tumoral Cancer Therapies Companies for the upcoming emerging drugs are INT230-6 (Intensity Therapeutics), Ilixadencel (INTUVAX) (Mendus), and others. The drug chapter also helps understand the Intra-Tumoral Cancer Therapies Clinical Trials details, expressive pharmacological action, agreements and collaborations, approval and patent details, and the latest news and press releases.
Intra-Tumoral Cancer Therapies Marketed Drugs
- IMLYGIC (talimogene laherparepvec): Amgen
IMLYGIC is a genetically modified herpes simplex virus type 1 (HSV-1) designed to replicate within tumors and produce an immunostimulatory protein called GM-CSF. IMLYGIC causes cell lysis, or death, which ruptures tumors, releasing tumor-derived antigens, which along with GM-CSF, may promote an antitumor immune response. However, the exact mechanism of action is unknown.
- In June 2023, Amgen announced multiple presentations of IMLYGIC for the treatment of resectable early metastatic (stage 3B/C/D-IV M1a) melanoma with injectable disease (NIVEC trial); cutaneous lymphomas and non-melanoma skin cancers; metastatic, unresectable, or locoregionally recurrent HER2-negative breast cancer; and results from the expansion cohorts of advanced sarcoma, at ASCO 2023.
- In October 2015, Amgen announced that the US FDA approved the BLA for IMLYGIC for the local treatment of unresectable cutaneous, subcutaneous, and nodal lesions in patients with recurrent melanoma after initial surgery. IMLYGIC is the first oncolytic viral therapy approved by the FDA based on therapeutic benefits demonstrated in a pivotal study
- In December 2015, Amgen announced that the European Commission approved the use of IMLYGIC for the treatment of adults with unresectable melanoma that is regionally or distantly metastatic (stage 3B, 3C, and IVM1a) with no bone, brain, lung, or other visceral diseases
- DELYTACT (teserpaturev): Daiichi Sankyo
DELYTACT, jointly developed by Daiichi Sankyo and the University of Tokyo’s Institute of Medical Science, is a genetically engineered oncolytic herpes simplex virus type 1 (HSV-1). DELYTACT has triple mutation within the viral genome that causes augmented and selective replication in cancer cells and enhanced induction of antitumor immune response while retaining high safety features. DELYTACT is currently the first third-generation oncolytic HSV-1 to be evaluated in humans. In June 2021, Daiichi Sankyo received conditional and time-limited approval from MHLW Japan for DELYTACT for treating patients with malignant glioma (Daiichi Sankyo, 2021b). In November 2021, Daiichi Sankyo announced the launch of DELYTACT in Japan (Daiichi Sankyo, 2021a).
Intra-Tumoral Cancer Therapies Emerging Drugs
- INT230-6: Intensity Therapeutics
INT230-6 is a formulation consisting of our proprietary amphiphilic cell penetration enhancer molecule, 8-(2-hydroxybenzoyl)amino)octanoate, also referred to as SHAO, combined with cisplatin and vinblastine. The penetration enhancer facilitates the dispersion of the two drugs throughout injected tumors and enables increased diffusion into cancer cells. Nonclinical safety studies showed no evidence of the drug penetrating healthy tissue. INT230-6 is currently being studied in multiple Phase II clinical cohorts to treat several types of metastatic cancers in the US and Canada.
- Ilixadencel (INTUVAX): Mendus
Ilixadencel is an off-the-shelf intratumoral immune primer consisting of pro-inflammatory monocyte-derived Dendritic Cells (DCs) from allogeneic healthy donor material. Upon administration, ilixadencel promotes a local pro-inflammatory environment, activation of NK cells, and cross-presentation of tumor antigens by recruited and activated endogenous DCs in the tumor microenvironment. In Intra-Tumoral Cancer Therapies Clinical Trials, ilixadencel has demonstrated promising signs of efficacy in hard-to-treat solid tumors and was shown to be safe in combination with tyrosine kinase inhibitors and immune checkpoint inhibitors. The data presented at SITC support the clinical evaluation of ilixadencel in combination with avelumab, the immune checkpoint inhibitor used in the REGOMUNE trial.
Ilixadencel comprises activated donor monocyte-derived DCs administered via intratumoral injection. Upon administration, ilixadencel triggers local immune activation and cross-presentation of tumor antigens. Intratumoral immune priming has the potential to turn “cold” tumors “hot” and more susceptible to the immune system
Note: Detailed emerging therapies assessment will be provided in the final report.
Intra-Tumoral Cancer Therapies Drugs Market Insights
The landscape of Intra-Tumoral Cancer Therapies Drugs development involves Angiogenesis inhibitors, Protein synthesis inhibitors, TGF-ß beta inhibitors etc. Angiogenesis inhibitors blocking the formation of new blood vessels around tumors, thereby starving them of oxygen and nutrients and hindering their growth and spread. directly blocking VEGF or its receptors, preventing them from activating the signaling pathways that lead to new blood vessel formation.
Intra-Tumoral Cancer Therapies Market Outlook
Cancer has been a focal point for research and development, and with time, the treatment paradigm has improved significantly. Cancer treatment mainly comprises surgery, radiation therapy, chemotherapy, hormone therapy, targeted therapy, immunotherapy, and stem cell transplantation. However, despite several treatment options, death rates are relatively high as these therapies have limitations and cannot target every patient. Few cancer therapies face the challenge of accessing targets deep within tumor tissue; some cause serious AEs.
Cancer patients have multiple treatment-related unmet needs, so researchers are working on developing intra-tumoral . Intra-tumoral (intralesional) immunotherapeutic delivery is a compelling solution to address local impediments to tumor immunity. Even though intra-tumoral therapy most factually means injecting directly into tumors, it can refer to any therapy delivered in close anatomical proximity with the intention of direct uptake by tumor cells
The US FDA approved IMLYGIC (T-Vec; Amgen), the first oncolytic virus, for treating unresectable melanoma lesions after surgery, based on a Phase III trial. The European Commission followed with approval in 2015 for advanced melanoma. Nanobiotix’s HENSIFY (NBTXR3), a hafnium oxide nanoparticle therapy combined with radiation, received European approval in 2019 for soft tissue sarcoma. The company plans data releases through 2025–2026 across various cancers. In Japan, DELYTACT (G47?; Daiichi Sankyo) received conditional approval for malignant glioma, the first oncolytic virus for brain cancer, with ongoing evaluation of its clinical benefit.
Globally, Intra-Tumoral Cancer Therapies Companies are advancing Intra-Tumoral Cancer Therapies. Philogen and Replimune are in Phase III, while Replimune's RP2 is in Phase II/III. Others in Phase II include Highlight Therapeutics, Cytovation ASA, Intensity Therapeutics, Ascendis Pharma, Treovir, QBiotics, NanOlogy, EpicentRx, Medicenna Therapeutics, and more.
Intra-Tumoral Cancer Therapies Drugs Uptake
This section focuses on the uptake rate of potential Intra-Tumoral Cancer Therapies drugs expected to be launched in the market during 2025–2034, which depends on the competitive landscape, safety, efficacy data, and order of entry. It is important to understand that the key Intra-Tumoral Cancer Therapies Companies evaluating their novel therapies in the pivotal and confirmatory trials should remain vigilant when selecting appropriate comparators to stand the greatest chance of a positive opinion from regulatory bodies, leading to approval, smooth launch, and rapid uptake.
Replimune has outlined its strategy for an independent commercial launch of RP1 in the second half of 2025. The US FDA has accepted the Biologics License Application (BLA) for RP1 in combination with nivolumab for the treatment of advanced melanoma.
Further detailed analysis of emerging therapies drug uptake in the report…
Intra-Tumoral Cancer Therapies Pipeline Development Activities
The Intra-Tumoral Cancer Therapies Therapeutics Market Report provides insights into different therapeutic candidates in Phase III and Phase II stages. It also analyzes key Intra-Tumoral Cancer Therapies Companies involved in developing targeted therapeutics.
Pipeline Development Activities
The Intra-Tumoral Cancer Therapies Therapeutics Market Report covers information on collaborations, acquisitions and mergers, licensing, and patent details for Intra-Tumoral Cancer Therapies emerging therapies.
KOL Views
To keep up with the real-world scenario in current and emerging market trends, we take opinions from Key Industry leaders working in the domain through primary research to fill the data gaps and validate our secondary research. Industry experts contacted for insights on the evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake along with challenges related to accessibility, including Medical/scientific writers, Professors, and others.
Delveinsight’s analysts connected with 20+ KOLs to gather insights; however, interviews were conducted with 10+ KOLs in the 7MM. Centers such as Miami Cancer Institute, Anderson Cancer Center, etc., were contacted. Their opinion helps understand and validate current and emerging therapy treatment patterns or Intra-Tumoral Cancer Therapies market trends.
Intra-Tumoral Cancer Therapies Therapeutics Market: Qualitative Analysis
We perform qualitative and market Intelligence analysis using various approaches, such as SWOT analysis and Analyst views. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the Analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving treatment landscape.
Intra-Tumoral Cancer Therapies Market Access and Reimbursement
Market Access refers to the ability of all patients to have access to a given product quickly, conveniently, and affordably. Reimbursement is the negotiation of a price between the manufacturer and payer that allows the manufacturer access to that market. It is provided to reduce the high costs and make essential drugs affordable.
Genentech has created a portal for the access and reimbursement of its products, named Genentech Access Solutions. It assists people after they have been prescribed Genentech medicines to manage the challenges that come with care. In case of a patient’s health insurance plan denial, the BioOncology Field Reimbursement Manager (BFRM) or AVASTIN Access Solutions Specialist can provide resources to prepare an appeal submission per the patient’s plan requirements. Genentech Access Solutions has also generated certain billing codes for its products, including AVASTIN.
Upcoming 2025 ASCO Annual Meeting Presentation updates
- NANOBIOTIX expects to provide the first data from Cohort 3 of the Study 1100 dose expansion part (advanced cancers other than R/M-HNSCC with lung, liver, or soft tissue metastases) in 2025.
- In June 2024, Ascendis Pharma reported new and updated results from its ongoing Phase I/II IL-Believe Trial of TransCon IL-2 ß/? in a poster presentation at ASCO 2024.
Intra-Tumoral Cancer Therapies Therapeutics Market Report Scope
- The Intra-Tumoral Cancer Therapies Therapeutics Market Report covers a segment of key events, an executive summary, and a descriptive overview, explaining its causes, signs and symptoms, pathogenesis, and currently available therapies.
- Comprehensive insight has been provided into the epidemiology segments and forecasts, the future growth potential of diagnosis rate, disease progression, and treatment guidelines.
- Additionally, an all-inclusive account of the current and emerging therapies and the elaborative profiles of late-stage and prominent therapies will impact the current Intra-Tumoral Cancer Therapies Treatment Market Landscape.
- A detailed review of the Intra-Tumoral Cancer Therapies Treatment Market, historical and forecasted Intra-Tumoral Cancer Therapies market size, market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The Intra-Tumoral Cancer Therapies Therapeutics Market Report provides an edge while developing business strategies by understanding trends through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help in shaping and driving the 7MM Intra-Tumoral Cancer Therapies Drugs Market.
Intra-Tumoral Cancer Therapies Therapeutics Market Report Insights
- Patient-based Intra-Tumoral Cancer Therapies Market Forecasting
- Therapeutic Approaches
- Intra-Tumoral Cancer Therapies Pipeline Drugs Analysis
- Intra-Tumoral Cancer Therapies Market Size and Trends
- Existing and Future Intra-Tumoral Cancer Therapies Drugs Market Opportunities
Intra-Tumoral Cancer Therapies Therapeutics Market Report Key Strengths
- 10 Years Intra-Tumoral Cancer Therapies Market Forecast
- The 7MM Coverage
- Intra-Tumoral Cancer Therapies Epidemiology Segmentation
- Key Cross Competition
- Drugs Uptake and Key Intra-Tumoral Cancer Therapies Market Forecast Assumptions
Intra-Tumoral Cancer Therapies Therapeutics Market Report
- Current Intra-Tumoral Cancer Therapies Treatment Practices
- Intra-Tumoral Cancer Therapies Unmet Needs
- Intra-Tumoral Cancer Therapies Pipeline Drugs Profiles
- Intra-Tumoral Cancer Therapies Drugs Market Attractiveness
- Qualitative Analysis (SWOT)
FAQs
- What is the historical and forecasted Intra-Tumoral Cancer Therapies patient pool in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan?
- What was the Intra-Tumoral Cancer Therapies Market Size, the market size by therapies, Intra-Tumoral Cancer Therapies Drugs Market Share (%) distribution in 2020, and what would it look like by 2034? What are the contributing factors for this growth?
- Which therapy is going to be the largest contributor by 2034?
- What are the pricing variations among different geographies for approved and off-label therapies?
- What are the recent novel therapies, targets, Intra-Tumoral Cancer Therapies Mechanisms of Action, and technologies developed to overcome the limitations of existing therapies?
- Patient acceptability in terms of preferred treatment options as per real-world scenarios?
- What are the country-specific accessibility issues of expensive, recently approved therapies?
Reasons to Buy
- The Intra-Tumoral Cancer Therapies Therapeutics Market Report will help in developing business strategies by understanding the latest trends and changing treatment dynamics driving the Intra-Tumoral Cancer Therapies Drugs Market.
- Insights on patient burden/disease cases, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years.
- Understand the existing Intra-Tumoral Cancer Therapies Drugs Market opportunities in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
- Identifying strong upcoming Intra-Tumoral Cancer Therapies Companies in the market will help devise strategies to help get ahead of competitors.
- Highlights of access and reimbursement policies of approved therapies, barriers to accessibility of expensive off-label therapies, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing market so that the upcoming Intra-Tumoral Cancer Therapies Companies can strengthen their development and launch strategy.
Stay updated with us for Recent Articles