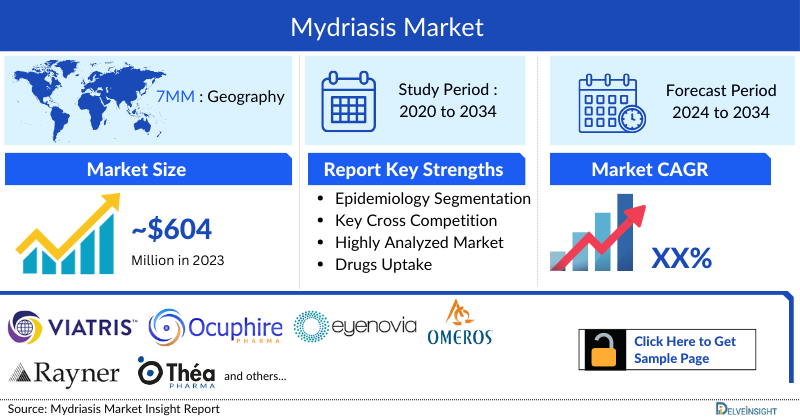
Mydriasis Market
- The Mydriasis market is set for steady growth, with a compound annual growth rate (CAGR) expected to remain strong from 2024 to 2034. This Mydriasis market growth will be driven primarily by the increase in age-related eye conditions such as cataracts, glaucoma, and macular degeneration, leading to more eye exams and surgeries.
- Key Mydriasis companies such as Omeros Corporation, Ocuphire Pharma, and others are working to develop therapies which will drive the Mydriasis market.
- The increasing Mydriasis prevalence of chronic conditions such as diabetes and hypertension in the US may lead to more frequent eye examinations to monitor for eye complications, which typically involve dilation.
- The current Mydriasis treatment market faces several key challenges, including the limited availability of effective and safe treatments, especially for long-term use. There is a lack of targeted therapies addressing underlying causes and insufficient research and development of novel therapeutic agents. Improved diagnostic tools to accurately identify the type and severity of Mydriasis are also needed.
- Currently, there is only one approved drug on the Mydriasis market, RYZUMVI, specifically tailored to rapidly reverse pharmacologically induced Mydriasis, highlighting a lack of Mydriasis treatment options for patients who seek to resume normal activities promptly following dilation.
- Currently, there are no emerging Mydriasis drugs in development specifically targeting pharmacologically induced Mydriasis, highlighting a gap in Mydriasis treatment options for individuals affected by this condition.
Key Factors Driving Mydriasis Market
Mydriasis Rising Prevalence
In 2024, the Mydriasis market is projected for steady growth, driven by the rising prevalence of age-related eye conditions such as cataracts, glaucoma, and macular degeneration, which increase the frequency of eye exams and ophthalmic surgeries. Chronic conditions like diabetes and hypertension further contribute to higher demand for pupil dilation during eye monitoring, particularly in the US. In 2023, eye exams accounted for approximately 338 million cases in the 7MM, while cataract surgeries totaled 8.6 million, glaucoma surgeries 538K, refractive surgeries 1.6 million, vitreoretinal surgeries 505K, and other procedures 2.9 million.
Mydriasis Treatment Landscape
Currently, Mydriasis treatment is largely procedural or symptomatic, focusing on reversing pharmacologically induced dilation. Key marketed therapies include RYZUMVI (phentolamine ophthalmic solution 0.75%) for rapid reversal of Mydriasis, MYDCOMBI (tropicamide + phenylephrine) for short-term dilation during diagnostics, and OMIDRIA (phenylephrine + ketorolac) to maintain Mydriasis during cataract surgery. Topical eye drop formulations dominate the market, generating revenues of USD 286.2 million for surgery applications and USD 183.5 million for diagnostic exams in 2023 across the 7MM, with OMIDRIA contributing USD 112.1 million.
Mydriasis Clinical Trials and Competitive Landscape
The Mydriasis market is currently limited in pipeline activity, with no emerging drugs specifically targeting pharmacologically induced Mydriasis. Key companies such as Omeros Corporation and Ocuphire Pharma continue to focus on optimizing existing therapies and procedural applications, reflecting a competitive landscape centered on maximizing the utility of approved agents like RYZUMVI, MYDCOMBI, and OMIDRIA.
DelveInsight’s “Mydriasis – Market Insights, Epidemiology, and Market Forecast – 2034” report delivers an in-depth understanding of Mydriasis, historical and forecasted epidemiology, as well as the Mydriasis market trends in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
The Mydriasis market report provides current treatment practices, emerging drugs, market share of individual therapies, and current and forecasted 7MM Mydriasis market size from 2020 to 2034. The report also covers Mydriasis treatment practices/algorithms and unmet medical needs to curate the best opportunities and assess the mydriasis treatment market’s potential.
Mydriasis Understanding and Treatment Algorithm
Mydriasis is defined as the abnormal dilation of the pupils, which can occur due to various non-physiological causes. Normally, pupils dilate in low light conditions to enhance vision and constrict in bright light to protect the retina. However, Mydriasis persists even in bright environments, indicating an underlying issue. This condition can occur in response to various stimuli, including low light, but in Mydriasis, the dilation persists even in bright environments.
The condition arises from the disruption of the normal balance between the sympathetic and parasympathetic nervous systems. The sympathetic system causes dilation, while the parasympathetic system, primarily through the oculomotor nerve (cranial nerve III), controls constriction. Damage to the oculomotor nerve or the iris sphincter can lead to Mydriasis, often seen in cases of traumatic brain injury, increased intracranial pressure, or specific neurological disorders. Additionally, certain medications, particularly anticholinergics and recreational drugs like cocaine and ecstasy, can induce Mydriasis.
Symptoms often include light sensitivity (photophobia), blurred vision, and discomfort in bright environments. Individuals may also experience headaches and eye pain, particularly if the dilation is linked to underlying conditions such as trauma or neurological issues. In severe cases, fixed unilateral Mydriasis could indicate raised intracranial pressure, necessitating immediate medical attention.
Mydriasis diagnosis
Mydriasis can be diagnosed through physical examination and medical history. Given the potential neurological implications of Mydriasis, a neurological examination is crucial. Signs of neurological compromise, such as altered consciousness or motor deficits, warrant immediate imaging studies like CT or MRI to assess for intracranial pathology.
Further details related to country-based variations are provided in the report…
Mydriasis treatment
Mydriasis treatment depends on the cause. If it is medication-induced, the doctor may recommend stopping the drug or finding an alternative. For eye injuries, Mydriasis treatment may involve addressing the underlying trauma. In some instances, topical agents like pilocarpine may be used to constrict the pupil.
Currently, RYZUMVI is the only US FDA-approved product available to rapidly reverse pharmacologically induced Mydriasis. Unlike other treatments, RYZUMVI specifically reverses dilation caused by adrenergic agonists and parasympatholytic agents without affecting the ciliary muscle.
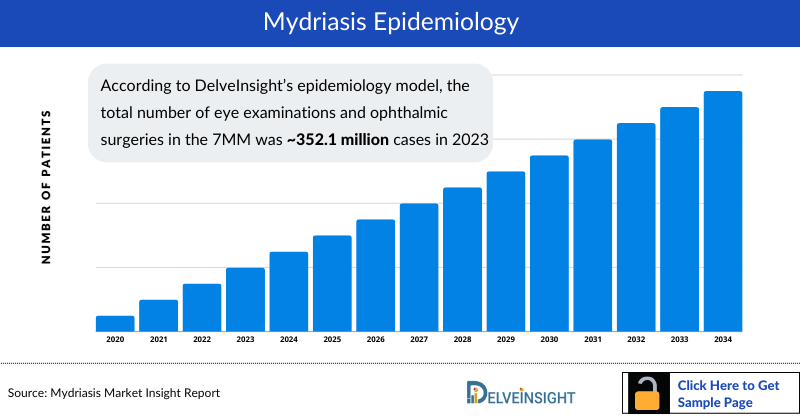
Mydriasis Epidemiology
As the Mydriasis market is derived using a patient-based model, the Mydriasis epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by total cases of eye examinations and ophthalmic surgeries and total cases of dilation in eye examinations and ophthalmic surgeries in the 7MM covering the United States, EU4 countries (Germany, France, Italy, and Spain) and the United Kingdom, and Japan from 2020 to 2034.
- According to DelveInsight’s epidemiology model, the total number of eye examinations and ophthalmic surgeries in the 7MM was approximately 352.1 million cases in 2023 which is expected to increase during the forecast period (2024-2034) due to the rise of age-related eye conditions such as cataracts, glaucoma, and macular degeneration increases, leading to more eye exams and surgeries.
- Of the total number of eye examinations and ophthalmic surgeries, eye exams and check-ups accounted for approximately 338.0 million cases in the 7MM in 2023. There were approximately 8.6 million cases of cataract surgery, 538 thousand cases of glaucoma surgery, 1.6 million cases of refractive surgery, 505 thousand cases of vitreoretinal surgery, and 2.9 million cases of other types of surgeries.
- The US accounted for 53%, while EU4 and the UK represented 32%, and Japan comprised 15% of the total cases of eye examinations and ophthalmic surgeries in 2023.
- In 2023, there were approximately 65.1 million cases of pupil dilation for eye examinations and nearly 5.1 million cases of pupil dilation for eye surgeries in EU4 and the UK.
- Among the EU4 and the UK, Germany had the highest number of pupil dilation cases for both eye examinations and surgeries in 2023, with 16.7 million and 1.5 million cases respectively followed by France with approximately 13.5 million pupil dilation cases for eye examinations and 1.3 million pupil dilation cases for ophthalmic surgeries. On the other hand, Spain had the lowest numbers, with 9.3 million pupil dilation cases for eye examinations and 634 thousand pupil dilation cases for ophthalmic surgeries.
- With a growing focus on preventive healthcare, more people will proactively seek eye exams to catch potential issues early, leading to increased dilation cases in EU4 and the UK, the total cases of dilation in eye examinations and surgeries are expected to increase.
- In Japan, there were approximately 29.7 million cases of pupil dilation for eye examinations and nearly 2.5 million cases of pupil dilation for eye surgeries in 2023. The Mydriasis epidemiology is expected to change during the forecast period.
Mydriasis Drugs
Mydriasis Marketed Drugs
RYZUMVI (Phentolamine Ophthalmic Solution 0.75%): Viatris/Ocuphire Pharma
RYZUMVI (previously Nyxol) is an anti-microbial, preservative-free, topical eye drop formulation of phentolamine ophthalmic solution 0.75%. It is the first and only relatively non-selective alpha-1 and alpha-2 adrenergic antagonist approved to reverse pharmacologically-induced Mydriasis. The drug is administered into each dilated eye following the completion of the ophthalmic examination or procedure to reverse Mydriasis. The onset of action after administration of RYZUMVI generally occurs in 30 min, with the maximal effect seen in 60–90 min and the effect lasting at least 24 h.
In September 2023, the US FDA approved RYZUMVI (phentolamine ophthalmic solution 0.75%) for the treatment of reverse pharmacologically induced Mydriasis.
MYDCOMBI: Eyenovia
MYDCOMBI by Eyenovia is a sterile ophthalmic spray combining tropicamide (1%) and phenylephrine hydrochloride (2.5%) for pupil dilation. Designed for easy administration, it utilizes the optejet dispenser to deliver precise microdroplets, enhancing comfort and reducing overflow risks compared to traditional eye drops. MYDCOMBI is indicated for diagnostic eye examinations and short-term Mydriasis.
In May 2023, the US FDA approved MYDCOMBI for inducing Mydriasis for diagnostic procedures and in situations where short-term pupil dilation is required.
OMIDRIA: Rayner Surgical/Omeros
OMIDRIA is an intra-ocular solution of phenylephrine and ketorolac at concentrations of 1% and 0.3%, respectively, and is prescribed for use during cataract surgery. Its primary function is to ensure that the pupil remains dilated throughout the procedure, thereby enhancing surgical visibility. Additionally, it plays a role in minimizing eye pain and reducing inflammation following surgery. This sterile solution is crucial for maintaining optimal conditions during the surgical process and promoting a smoother recovery period for patients undergoing cataract surgery.
The US FDA approved OMIDRIA for use during cataract surgery or intra-ocular lens replacement procedures to maintain pupil size by preventing intra-operative miosis in May 2014 and it was approved in Europe for use in cataract and lens replacement surgery to maintain Mydriasis in August 2018.
Drug Class Insights
REV-EYES (dapiprazole hydrochloride), initially approved in 1990, is an alpha-1 adrenergic antagonist used to reverse Mydriasis after eye examinations by inducing pupil constriction. Despite its initial approval in 1990, it faced discontinuation due to limited clinical benefits compared to other treatments and a lack of significant demand. In 2013, the US FDA confirmed that Rev-Eyes was not officially withdrawn, but its distribution remained scarce.
RYZUMVI (phentolamine ophthalmic solution) 0.75% is a novel Mydriasis treatment for reversing Mydriasis, approved by the US FDA in September 2023. Unlike Rev-Eyes, which used dapiprazole, RYZUMVI is a preservative-free formulation that acts as a nonselective alpha-1 and alpha-2 adrenergic antagonist, providing a faster onset of action (30 min) and longer effect duration. Its unique dual mechanism allows it to counteract dilation from various mydriatic agents effectively. RYZUMVI represents a major advancement in addressing the challenges associated with pupil dilation, enabling broader access to eye examinations and improved eye health outcomes. As an alpha-adrenergic antagonist, RYZUMVI introduces an innovative and highly potent strategy for reversing drug-induced Mydriasis. RYZUMVI's exceptional properties encompass its rapid onset of action, with maximal efficacy achieved.
Cholinergic agents, particularly pilocarpine, are commonly used to induce pupillary constriction (miosis) by stimulating the parasympathetic nervous system. Pilocarpine mimics acetylcholine, a neurotransmitter that activates the iris sphincter muscle, leading to rapid pupil contraction. This effect is particularly valuable in clinical settings, such as during eye examinations, where restoring normal pupil size is essential for accurate assessment. Additionally, pilocarpine is effective in managing adverse reactions to mydriatic medications, which can cause prolonged pupil dilation (Mydriasis). By facilitating the swift reversal of Mydriasis, pilocarpine enhances patient comfort and safety in ophthalmic care.
Dapiprazole is an alpha-adrenergic blocker that effectively reduces Mydriasis caused by drugs like tropicamide and phenylephrine. By blocking alpha-adrenergic receptors in the smooth muscle of the iris, dapiprazole induces miosis (pupil constriction) by inhibiting the dilator muscle's action. This mechanism offers a rapid and safe alternative to cholinergic agents for reversing drug-induced pupil dilation, making it beneficial in clinical settings where quick restoration of average pupil size is necessary.
The absence of new Mydriasis therapies and Mydriasis drugs specifically designed to reverse pharmacologically induced Mydriasis can largely be attributed to the recent approval of RYZUMVI (phentolamine ophthalmic solution), which has effectively addressed this treatment gap. Before RYZUMVI, options for reversing Mydriasis were limited, which may have discouraged further research and development in this area. Additionally, the relatively low prevalence and duration of pharmacologically induced Mydriasis may not warrant the significant investment needed for developing new treatments. The interplay of market conditions and regulatory hurdles likely contributes to the slow emergence of additional therapies for this indication.
Continued in report…
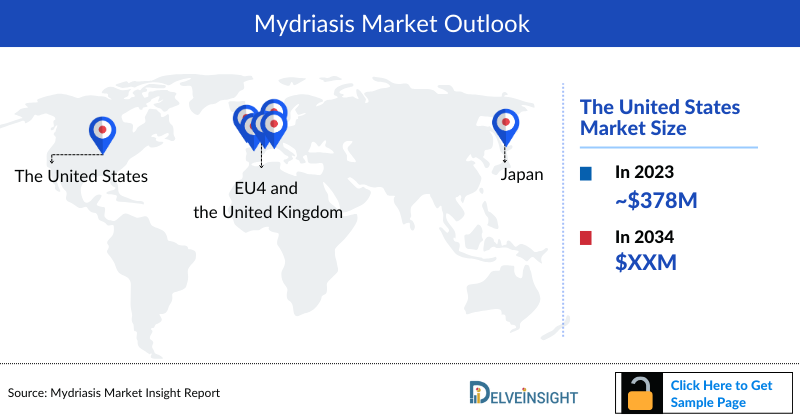
Mydriasis Market Outlook
Mydriasis, defined as the abnormal dilation of the pupils, can occur due to various factors, including pharmacological agents, neurological conditions, or trauma. The primary goal of treatment for Mydriasis is to restore the pupil to its normal size, alleviate associated symptoms such as light sensitivity and blurred vision, and address any underlying causes.
The Mydriasis treatment approach is contingent upon its underlying cause. The first step in managing Mydriasis is to identify its cause.
The Mydriasis treatment patterns vary across several countries, with specific mydriatic agents being preferred. Some common mydriatic agents include tropicamide, phenylephrine, and MYDRIASERT. In the US, Phenylephrine (1.0%) is favored, especially in surgical scenarios, due to its quick onset and brief effect duration. Similarly, in the UK and Germany, these agents are chosen for their efficacy in achieving pharmacologically induced Mydriasis, which is essential for diagnostic and therapeutic procedures. These agents, such as tropicamide and phenylephrine, act on specific receptors in the iris muscles. Tropicamide, for instance, blocks the action of acetylcholine at muscarinic receptors, causing relaxation of the iris sphincter muscle and, thus, dilation of the pupil. Phenylephrine, on the other hand, stimulates alpha-adrenergic receptors, leading to the contraction of the dilator muscle of the iris, which results in pupil dilation. Together, these mechanisms allow these agents to induce pharmacological Mydriasis, enlarging the pupil for clinical examinations and procedures.
Certain medications, particularly anticholinergics (e.g., atropine, scopolamine) and stimulants (e.g., cocaine, amphetamines), can also induce Mydriasis. If Mydriasis is drug-induced, discontinuation of the offending medication is often recommended, as symptoms may resolve on their own within hours or days. Reversal of pharmacologically induced Mydriasis eye drops can be used to speed up the return to standard pupil size, restore normal vision, and reduce light sensitivity so there is less disruption to daily activities. Mydriasis can indicate severe conditions such as third nerve palsy or increased intracranial pressure. In these cases, urgent medical evaluation is necessary to determine the appropriate treatment, which may include surgical intervention if structural damage is present. In some cases, medications such as pilocarpine and alpha-blockers such as dapiprazole, typically used for glaucoma, may be prescribed to help constrict the pupils.
The Mydriasis treatment is multifaceted, focusing on identifying and addressing the underlying cause while managing symptoms to improve patient comfort.
- The total Mydriasis market size in the 7MM was approximately USD 604.4 million in 2023 and is projected to increase during the forecast period (2024–2034).
- The total Mydriasis market sizein the US was approximately USD 378.6 million in 2023, accounting for approximately 63% of the total Mydriasis market revenue for the 7MM.
- The total Mydriasis market size in EU4 and the UK was calculated to be approximately USD 150.4 million in 2023. Among the EU4 and the UK, Germany accounted for the highest market with approximately USD 42.7 million, followed by France with approximately USD 36.5 million in the respective year, and the UK with USD 28.3 million.
- The total Mydriasis market size in Japan was approximately USD 75.4 million in 2023.
- Among the currently used Mydriasis therapies, the majority of the Mydriasis market share was of topical (eye drops/irrigation) for surgeries, with a revenue of approximately USD 286.2 million in 2023 among the 7MM followed by topical (eye drops/irrigation) for eye exams with a revenue of approximately USD 183.5 million, and OMIDRIA with a revenue of approximately USD 112.1 million.
Mydriasis Drugs Uptake
This section focuses on the uptake rate of potential Mydriasis drugs expected to be launched in the market during 2020–2034.
Further detailed analysis of emerging therapies drug uptake in the report…
Mydriasis Pipeline Development Activities
The report provides insights into different therapeutic candidates. It also analyzes key Mydriasis companies involved in developing targeted therapeutics.
Pipeline development activities
The report covers information on collaborations, acquisitions and mergers, licensing, and patent details for emerging therapies for Mydriasis.
KOL Views
To keep up with current market trends, we take KOLs and SMEs’ opinions working in the domain through primary research to fill the data gaps and validate our secondary research. Industry Experts contacted for insights on Mydriasis evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake, along with challenges related to accessibility, including Medical/scientific writers, Medical Professionals, Professors, Directors, and Others.
DelveInsight’s analysts connected with 50+ KOLs to gather insights; however, interviews were conducted with 15+ KOLs in the 7MM. Centers like the University of Texas, US, University of Texas, US, University of Texas, US, Universitäts-Augenklinik, Germany, University Hospital Necker Enfants-Malades, France, and Tokyo Women’s Medical University, Japan were contacted. Their opinion helps understand and validate current and emerging Mydriasis therapy treatment patterns or Mydriasis market trends. This will support the clients in potential upcoming novel Mydriasis treatments by identifying the overall scenario of the Mydriasis market and the unmet needs.
Physician’s View
Mydriasis is induced during eye exams and surgeries for better visualization, with recovery taking 3–8 hours, sometimes up to 24 hours. Treatment to reverse Mydriasis mainly uses pilocarpine. Annual dilated eye exams are crucial for those 65 and older, but under 60% of 93 million high-risk US adults receive them. Diagnosis involves physical exams, with pilocarpine showing little response in drug-induced cases. Dapiprazole can also reverse Mydriasis. There's a need for better reversal agents and more research on long-term effects, particularly for children and the elderly.
Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the Analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving treatment landscape.
Conjoint Analysis analyzes multiple emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy.
To analyze the effectiveness of these therapies, have calculated their attributed analysis by giving them scores based on their ability to improve atrial and ventricular dimension/function and ability to regulate heart rate.
Further, the therapies’ safety is evaluated wherein the adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials, which directly affects the safety of the molecule in the upcoming trials. It sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the route of administration, order of entry and designation, probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Market Access and Reimbursement
Nevertheless, several organizations in the 7MM are working to provide support to patients and spread awareness, including OMIDRIAssure coverage and reimbursement support services, among others.
OMIDRIA
The OMIDRIAssure coverage and reimbursement support services for surgeons and facilities remove uncertainties about coding, billing, and coverage of OMIDRIA. The “Equal Access” patient assistance and the “We Pay the Difference” commercial reimbursement programs remove patients’ financial barriers to accessing the drug. Under the “Equal Access” program, financially eligible uninsured and government-insured patients receive OMIDRIA free of charge for use during surgery. For commercially insured patients, Omeros, through its “We Pay the Difference” program, pays the facility, on behalf of the patient, the difference between the facility’s acquisition cost for OMIDRIA and the amount covered by the patient’s insurance less a USD 30 patient responsibility.
Reimbursement
Since October 2019, HCPCS code J1097 (Phenylephrine 10.16 mg/ml and ketorolac 2.88 mg/ml ophthalmic irrigation solution, 1 ml) has been used to describe the supply of the drug. Effective January 2021, CMS reimburses ASCs for OMIDRIA as a surgical supply item. As of April 2022, this payment was USD 98.53 per unit. The ASC reimbursement rate changes periodically based on the manufacturer’s average selling price of +6%, as reported to CMS.
For Part B Medicare beneficiaries, there was separate payment as a pass-through drug through September 2020 because OMIDRIA formerly qualified for pass-through under the Outpatient Prospective Payment System (OPPS) that governs reimbursement to facilities. The type of coverage changed for dates of service on or after October 2020; OMIDRIA now has Medicare coverage for ASCs as a non-opioid drug supplied for pain management.
Although Medicare coverage for OMIDRIA changed from being a new drug with pass-through status to non-opioid pain management, ASCs continue to report four units on claims as they have since October 2019 with this J-code. Use of the J-code does not guarantee payment.
In the hospital outpatient department (HOPD), under OPPS rules, payment for the drug is included in the facility reimbursement, not separate.
Further details will be provided in the report.
The report provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenarios, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Scope of the Report
- The report covers a segment of key events, an executive summary, and a descriptive overview of Mydriasis, explaining its causes, signs and symptoms, pathogenesis, and currently available therapies.
- Comprehensive insight into the epidemiology segments and forecasts, the future growth potential of diagnosis rate, disease progression, and treatment guidelines have been provided.
- Additionally, an all-inclusive account of the current and emerging therapies and the elaborative profiles of late-stage and prominent therapies will impact the current treatment landscape.
- A detailed review of the Mydriasis market, historical and forecasted market size, market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The report provides an edge while developing business strategies by understanding trends through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help shape and drive the 7MM Mydriasis market.
Mydriasis report insights
- Patient Population
- Therapeutic Approaches
- Mydriasis Pipeline Analysis
- Mydriasis Market Size and Trends
- Existing and Future Market Opportunity
Mydriasis report key strengths
- 11 years Forecast
- The 7MM Coverage
- Mydriasis Epidemiology Segmentation
- Key Cross Competition
- Attribute analysis
- Drugs Uptake and Key Market Forecast Assumptions
Mydriasis report assessment
- Current Treatment Practices
- Unmet Needs
- Pipeline Product Profiles
- Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
Key Questions
Market Insights
- What was the total market size of Mydriasis, the market size of Mydriasis by therapies, and market share (%) distribution in 2020, and what would it look like by 2034? What are the contributing factors for this growth?
- How will RYZUMVI affect the treatment paradigm of Mydriasis?
- How will RYZUMVI compete with other marketed therapies?
- Which drug is going to be the largest contributor by 2034?
- What are the pricing variations among different geographies for approved and marketed therapies?
- How would future opportunities affect the market dynamics and subsequent analysis of the associated trends?
Epidemiology Insights
- What are the disease risks, burdens, and unmet needs of Mydriasis? What will be the growth opportunities across the 7MM with respect to the patient population pertaining to Mydriasis?
- What is the historical and forecasted Mydriasis patient pool in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan?
- What factors are contributing to the growth of Mydriasis cases?
Current Treatment Scenario, Marketed Drugs, and Emerging Therapies
- What are the current options for the treatment of Mydriasis? What are the current clinical and treatment guidelines for treating Mydriasis?
- How many companies are developing therapies for the treatment of Mydriasis?
- What are the recent novel therapies, targets, mydriasis mechanisms of action (mydriasis MOA), and technologies developed to overcome the limitations of existing therapies?
- What is the cost burden of current treatment on the patient?
- Patient acceptability in terms of preferred treatment options as per real-world scenarios?
- What are the accessibility issues of approved therapy in the US?
- What is the 7MM historical and forecasted market of Mydriasis?
Reasons to Buy
- The report will help develop business strategies by understanding the latest trends and changing treatment dynamics driving the Mydriasis market.
- Insights on patient burden/Mydriasis prevalence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years.
- Understand the existing market opportunities in varying geographies and the growth potential over the coming years.
- The distribution of historical and current patient share is based on real-world prescription data in the US, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
- Identifying upcoming solid players in the market will help devise strategies to help get ahead of competitors.
- Detailed analysis and ranking of class-wise potential of current and emerging therapies under the conjoint analysis section to provide visibility around leading classes.
- Highlights of Access and Reimbursement policies for Mydriasis, barriers to accessibility of approved therapy, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing market so that the upcoming players can strengthen their development and launch strategy.
Related Reports:



.png&w=256&q=75)


