Paroxysmal Nocturnal Hemoglobinuria Market Summary
- The Paroxysmal Nocturnal Hemoglobinuria market size in the 7MM is expected to grow from USD 1,554 million in 2025 to USD 2,883 million in 2034.
- The Paroxysmal Nocturnal Hemoglobinuria market is projected to grow at a CAGR of 7.1% by 2034 in leading countries like US, EU4, UK and Japan.
Paroxysmal Nocturnal Hemoglobinuria Market and Epidemiology Analysis
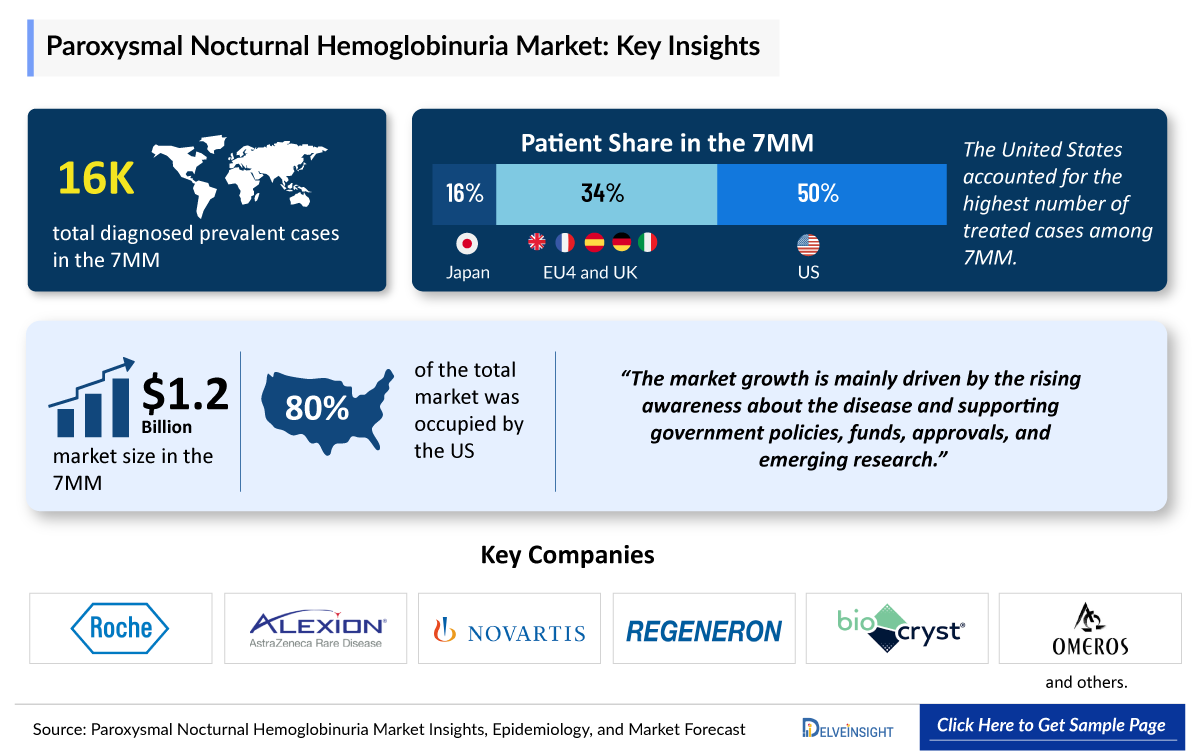
- The United States accounted for the highest Paroxysmal Nocturnal Hemoglobinuria Market Size among the 7MM around 85% of the total market size.
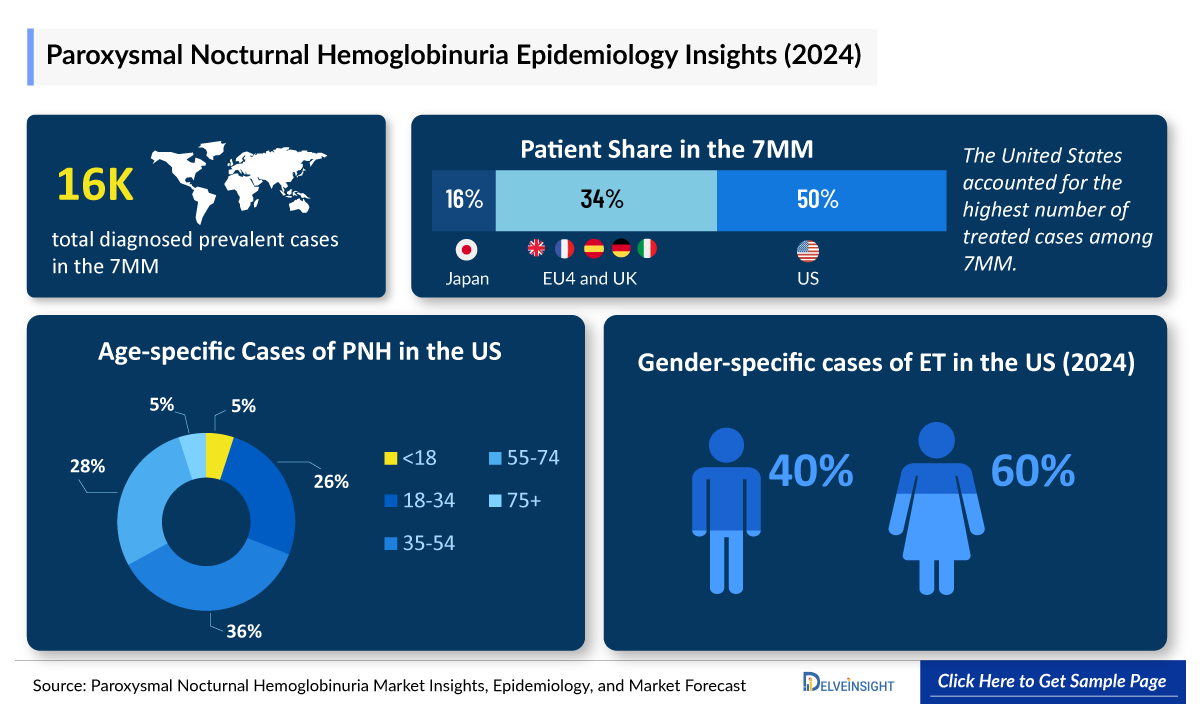
- In 2024, the Paroxysmal Nocturnal Hemoglobinuria Diagnosed Prevalent Cases were approximately 16,000 cases in the 7MM, which will increase by 2034. In the 7MM, the highest number of Paroxysmal Nocturnal Hemoglobinuria diagnosed prevalent cases were observed in the US.
- In the US, females were more affected by Paroxysmal Nocturnal Hemoglobinuria than males, in 2024.
- Among EU4 and the UK, in 2024, the UK accounted for the highest number of Paroxysmal Nocturnal Hemoglobinuria cases, followed by Germany, whereas Italy accounted for the lowest number of Paroxysmal Nocturnal Hemoglobinuria diagnosed prevalent cases.
- Paroxysmal Nocturnal Hemoglobinuria is a rare nonmalignant clonal hematological disorder that is characterized by a deficiency of the GPI-linked complement regulators on the membrane of hematopoietic cells, which renders them susceptible to complement-mediated damage.
- Formerly, Paroxysmal Nocturnal Hemoglobinuria Diagnosis relied on Ham and sucrose tests, now replaced by flow cytometry (FCM) detecting GPI-anchored protein anomalies using monoclonal antibodies or FLAER. FLAER is suitable for nucleated cells but not red blood cells due to glycophorin interference. Also, the lack of international guidelines for proper screening and diagnosis leads to treatment delays.
- Historically, Paroxysmal Nocturnal Hemoglobinuria Treatment was exclusively supportive, and the median survival of patients was 15–20 years, thrombosis being the major cause of death. Supportive measures included corticosteroids during hemolytic attacks, androgen therapy, and red blood cell transfusions for the treatment of anemia, as well as anticoagulant therapy to treat/prevent thrombotic events. However, the use of these palliative measures was not evidence-based, their effectiveness was limited, and they were not devoid of adverse events. Allogeneic HSC transplant was and continues to be, indeed, the only curative Paroxysmal Nocturnal Hemoglobinuria Treatment, but it is reserved for young patients with concurrent bone marrow failure because of transplant-related morbidity and mortality.
- Eculizumab, the first developed complement factor inhibitor, Complement C5 Inhibitors, blocking the terminal complement cascade. Given the benefits of the complement inhibition strategy, changing the natural history of this disease, several novel Paroxysmal Nocturnal Hemoglobinuria Drugs against C5 (terminal inhibitors) or factors upstream C5 (proximal inhibitors) have been developed with the aim of further improving the Paroxysmal Nocturnal Hemoglobinuria Treatment.
- The disease-modifying therapeutic strategy for PNH includes complement inhibition therapy, with Paroxysmal Nocturnal Hemoglobinuria Drugs like SOLIRIS, ULTOMIRIS, and EMPAVELI approved by the FDA, and considered the gold standard. However, safety concerns are associated with eculizumab and ravulizumab, including the risk of meningococcal infections and the persistence of extravascular hemolysis in some individuals treated with eculizumab.
Paroxysmal Nocturnal Hemoglobinuria Market size and forecast:
- 2025 Market Size: USD 1,554 million in 2025
- 2034 Projected Market Size: USD 2,883 million in 2034
- Growth Rate (2025-2034): 7.1% CAGR
- Largest Market: United States
Request for Unlocking the Sample Page of the "Paroxysmal Nocturnal Hemoglobinuria Treatment Market"
Key Factors Driving the Paroxysmal Nocturnal Hemoglobinuria (PNH) Market
Rising prevalence of PNH
Paroxysmal Nocturnal Hemoglobinuria is a rare, life-threatening hematologic disorder with an incidence of ~1–2 cases per million annually, translating to around 16K diagnosed prevalent cases in the 7MM in 2024. Improved flow cytometry techniques and broader clinical awareness are driving earlier and more accurate diagnosis, gradually expanding the treatable patient pool.
Current treatment in the PNH Market
The introduction of complement inhibitors transformed PNH care. SOLIRIS (eculizumab, Alexion/AstraZeneca) was the first therapy, followed by ULTOMIRIS (ravulizumab, AstraZeneca), offering longer dosing intervals. Supportive measures such as transfusions and anticoagulation remain adjuncts.
PNH competitive landscape
Pipeline activity is robust, with oral complement inhibitors like danicopan (AstraZeneca), iptacopan (Novartis), and crovalimab (Roche) leading the way. Other targets include proximal inhibitors (factor B/D blockade) and gene therapy approaches aimed at long-term disease modification.
|
Scope of the Paroxysmal Nocturnal Hemoglobinuria Market Report | |
|
Study Period |
2020–2034 |
|
Forecast Period |
2025–2034 |
|
Geographies Covered |
|
|
Epidemiology |
Segmented by: · Total Diagnosed Prevalent cases · Gender-specific cases · Age group-specific cases · Total Treated cases |
|
Market |
Segmented by: · Region · Therapies |
|
Market Analysis |
· KOL Views · SWOT Analysis · Reimbursement · Conjoint Analysis · Unmet needs |
Paroxysmal Nocturnal Hemoglobinuria Disease Understanding
Paroxysmal Nocturnal Hemoglobinuria is a rare acquired disorder that affects the pluripotent hematopoietic stem cells, which can impact erythrocytes, leukocytes, thrombocytes, and potentially some endothelial cells. This condition arises from a somatic mutation in the X-linked PIG-A gene, which is involved in synthesizing the glycosylphosphatidylinositol (GPI) anchor required for attaching certain proteins to the cell membrane.
The mutation leads to a deficiency in the GPI anchor, resulting in the underexpression of crucial proteins, such as the complement regulatory proteins 'decay-accelerating factor' (DAF or CD55) and 'membrane inhibitor of reactive lysis' (MIRL or CD59), on the surface of the hematopoietic stem cells and the cells derived from them. This deficiency makes red blood cells more susceptible to attack by the complement system, causing complement-mediated intravascular hemolysis. As a consequence, free hemoglobin levels in the plasma increase, which binds to nitric oxide, leading to its depletion.
Further details are provided in the report…
Paroxysmal Nocturnal Hemoglobinuria Diagnosis
PNH diagnosis was formerly comforted by in vitro complement activation by either acidity (Ham test) or osmolarity (sucrose test). These tests are obsolete as diagnosing PNH by flow cytometry (FCM) refers to detecting a pathognomonic anomaly. GPI-anchored proteins can be detected after labeling the cells with monoclonal antibodies (for example, anti-CD55 or anti-CD59) or a reagent known as fluorescein-tagged proaerolysin (FLAER), which binds to the glycan portion of the GPI anchor. FLAER is best used on nucleated cells; it does not stain red blood cells, as red blood cells express high glycophorin levels, a protein that binds to aerolysin and interferes with the assay.
Further details related to country-based variations are provided in the report…
Paroxysmal Nocturnal Hemoglobinuria Treatment
Treatment with a C5 inhibitor is the standard for patients with PNH. Eculizumab (SOLIRIS, Alexion Pharmaceuticals) and ravulizumab (ULTOMIRIS, Alexion Pharmaceuticals) are humanized monoclonal antibodies that block terminal complement activation at C5 and inhibit the formation of C5a and C5b. Eculizumab was approved by the US FDA in 2007, and ravulizumab in 2018. These agents bind to the same C5 epitope and have the same mechanism of action; ravulizumab was engineered from eculizumab to have a longer half-life. Both drugs are administered via IV infusion. Pegcetacoplan, the first C3 inhibitor for the Paroxysmal Nocturnal Hemoglobinuria Treatment, was also recently approved by the FDA and EMA.
Further details related to treatment and management are provided in the report…
Paroxysmal Nocturnal Hemoglobinuria Epidemiology
The Paroxysmal Nocturnal Hemoglobinuria epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by Total diagnosed prevalent cases, gender-specific cases, age-specific cases, and total treated cases in the United States, EU4 countries (Germany, France, Italy, Spain) and the United Kingdom, and Japan from 2020 to 2034.
Key Findings from Paroxysmal Nocturnal Hemoglobinuria Epidemiological Analysis and Forecast:
- The total Paroxysmal Nocturnal Hemoglobinuria Diagnosed Cases in the US were around 8,000 cases in 2024.
- In the United States, the highest number of age-specific cases were recorded for the 35-54 age group, followed by the age group of 55-74 yrs.
- The United Kingdom had the highest Paroxysmal Nocturnal Hemoglobinuria Diagnosed Prevalent Cases among the EU4 and the UK, accounting for more than 50% of cases in 2024.
- In 2024, the total Paroxysmal Nocturnal Hemoglobinuria Treated Cases in the US were around 5,000.
Paroxysmal Nocturnal Hemoglobinuria Epidemiology Segmentation:
- Total diagnosed prevalent cases
- Gender-specific cases
- Age-specific cases
- Total treated cases
Paroxysmal Nocturnal Hemoglobinuria Drugs Market Chapters
The section dedicated to drugs in the Paroxysmal Nocturnal Hemoglobinuria therapeutics market report provides an in-depth evaluation of late-stage Paroxysmal Nocturnal Hemoglobinuria pipeline drugs (Phase III and Phase II). The drug chapters section provides valuable information on various aspects related to Paroxysmal Nocturnal Hemoglobinuria Clinical Trials, such as the pharmacological mechanisms of the drugs involved, designations, approval status, patent information, and a comprehensive analysis of the pros and cons associated with each drug. Furthermore, it presents the most recent news updates and press releases on drugs targeting Paroxysmal Nocturnal Hemoglobinuria.
Paroxysmal Nocturnal Hemoglobinuria Marketed Therapies
-
ULTOMIRIS (ravulizumab): AstraZeneca (Alexion Pharmaceuticals)
ULTOMIRIS is a complement inhibitor for treating adult patients with PNH. It is designed to inhibit a specific aspect of the complement component of the immune system and thereby treat inflammation associated with chronic disorders in several therapeutic areas, including hematology, nephrology, and neurology.
In December 2018, the US FDA approved ULTOMIRIS (ravulizumab) for treating PNH. Following this, the European Union authorized it in July 2019, and Japan's MHLW approved it in June 2019 for PNH treatment. In June 2021, the US FDA expanded its approval to include children and adolescents (one month and older) with PNH.
-
EMPAVELI/ ASPAVELI (pegcetacoplan): Apellis Pharmaceuticals/ Swedish Orphan Biovitrum
Pegcetacoplan (APL-2) is an investigational, targeted C3 inhibitor designed to regulate excessive complement activation, leading to the onset and progression of many serious diseases. Pegcetacoplan is a synthetic cyclic peptide conjugated to a polyethylene glycol polymer that binds specifically to C3 and C3b. EMPAVELI is a complement inhibitor indicated to treat adult patients with PNH.
In April 2021, the FDA approved EMPAVELI (pegcetacoplan) injection to treat adults with PNH. EMPAVELI was the first PNH treatment that was bound to complement protein C3.
|
Comparison of Marketed Drugs | ||||||
|
Product |
Company |
Paroxysmal Nocturnal Hemoglobinuria MoA |
RoA |
Indication |
Molecule Type |
Initial Approval |
|
ULTOMIRIS (ravulizumab) |
Alexion Pharmaceuticals/AstraZeneca |
C5 complement inhibitor |
IV/SC |
PNH in patients 1 month age or older |
Monoclonal antibody |
US: 2018 EU: 2019 JP: 2019 |
|
EMPAVELI/ASPAVELI (Pegcetacoplan) |
Apellis pharmaceuticals |
C3 complement inhibitor |
SC |
PNH in adult patients |
Monoclonal antibody |
US: 2021 EU: 2021 JP:2023 |
|
FABHALTA (iptacopan) |
Novartis |
Complement factor B inhibitor |
Oral |
PNH |
Small molecule |
US:2023 EU: 2024 JP: 2024 |
Paroxysmal Nocturnal Hemoglobinuria Emerging Therapies
-
Pozelimab (REGN3918) + Cemdisiran: Regeneron Pharmaceuticals/Alnylam Pharmaceuticals
Pozelimab is an investigational, fully human monoclonal antibody designed to block complement factor C5 and prevent the destruction of red blood cells (hemolysis) that cause the symptoms of PNH and other diseases mediated by complement pathway activity. It is an IgG4 antibody that binds with high affinity to wild-type and variant human C5 and blocks its activity.
In August 2019, Regeneron Pharmaceutical’s Pozelimab was granted ODD by the FDA to treat PNH. The Company announced positive updated data from a Phase III study of pozelimab (C5 antibody), in combination with cemdisiran (siRNA therapy), against ravulizumab, a standard-of-care C5 inhibitor, in patients with Paroxysmal Nocturnal Hemoglobinuria.
- OMS-906 (zaltenibart)
OMS-906 (zaltenibart) is a humanized IgG4 monoclonal antibody that inhibits MASP-3, suppressing the alternative complement pathway upstream by blocking factor D activation. Developed by Omeros and acquired by Novo Nordisk in 2025, it has shown potent control of intra- and extravascular hemolysis in PNH with favorable Phase Ib results. The therapy is in Phase II development for PNH and glomerulonephritis.
-
Zaltenibart (OMS906): Omeros Corporation
Zaltenibart (OMS906) is a humanized monoclonal antibody targeting mannan-binding lectin-associated serine protease-3 (MASP-3), the key activator of the alternative pathway of complement. The alternative pathway is implicated in a wide range of diseases and disorders, including those targeted by marketed alternative pathway inhibitors and/or those in development.
In July 2022, Omeros Corporation announced that OMS906 had received ODD from the US FDA for the Paroxysmal Nocturnal Hemoglobinuria Treatment.
- NM-8074: NovelMed
NM-8074 (ruxoprubart) is a humanized monoclonal antibody from NovelMed Therapeutics that inhibits complement factor Bb, selectively blocking the alternative complement pathway while preserving classical and lectin pathways. In Phase II trials, it has demonstrated improved hemoglobin levels, reduced LDH, and transfusion independence in PNH patients. NM-8074 is also being evaluated for aHUS and dermatomyositis and remains investigational with orphan drug designation.
|
Comparison of Emerging Therapies | |||||||
|
Product |
Company |
Phase |
Indication |
Designation |
RoA |
Paroxysmal Nocturnal Hemoglobinuria MoA |
Molecule Type |
|
Pozelimab + Cemdisiran |
Regeneron Pharmaceuticals |
III |
PNH |
ODD |
IV/SC |
C5 complement inhibitor |
Monoclonal Antibody |
|
Zaltenibart (OMS906) |
Omeros Corporation |
II |
PNH |
ODD |
IV |
MASP-3 inhibitor |
Monoclonal Antibody |
|
Ruxoprubart (NM8074) |
NovelMed Therapeutics |
II |
PNH |
ODD |
IV |
Complement C3–C5 convertases inhibitor |
Monoclonal Antibody |
Note: Detailed assessment will be provided in the final report of PNH…
Paroxysmal Nocturnal Hemoglobinuria Market Outlook
Substantial progress in the biology and Paroxysmal Nocturnal Hemoglobinuria treatment has occurred over the past two decades, making PNH a model for progress in precision medicine. A thorough understanding of the molecular and cellular underpinnings of PNH has led to the development of a targeted therapy, which has altered the natural history of the disease. However, there is still room for improvement in caring for Paroxysmal Nocturnal Hemoglobinuria Patients. The future approvals of Paroxysmal Nocturnal Hemoglobinuria move toward personalized patient-centered care with better options to reduce the frequency and self-administer medication. Auto-injections of SC compounds and oral drugs will increase patient autonomy, and compliance will become critical.
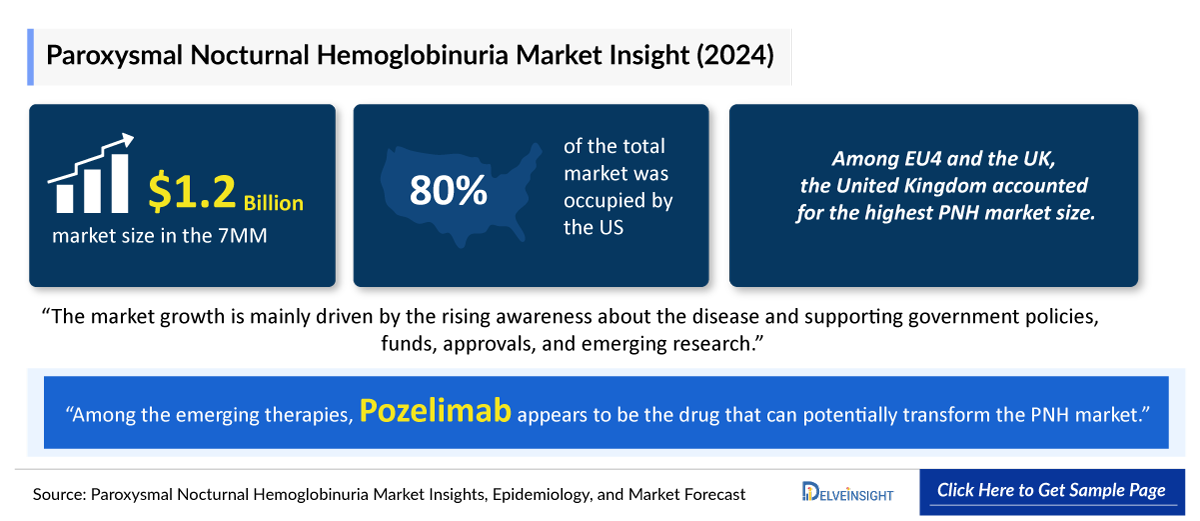
The US has the highest Paroxysmal Nocturnal Hemoglobinuria Drugs Market Share, followed by the EU4 and the UK, and Japan. Rising awareness about the disease and supporting government policies, funds, approvals, and emerging research would drive the market significantly in forecast periods (2025–2034).
Paroxysmal Nocturnal Hemoglobinuria Market Insights
- The leading Paroxysmal Nocturnal Hemoglobinuria Companies such as Alexion Pharmaceuticals, Novartis, Chugai Pharmaceuticals, Apellis Pharmaceuticals, and Others. The details of the country-wise and therapy-wise market size have been provided below.
- In the Paroxysmal Nocturnal Hemoglobinuria Treatment Market Size in the 7MM, the United States accounted for the highest market share, i.e., ~85% in 2024, followed by the United Kingdom.
- Among EU4 and the UK, the United Kingdom accounted for the highest Paroxysmal Nocturnal Hemoglobinuria Market Size in 2024.
- The United States accounted for more than USD 1,000 million in 2024.
- Among the emerging therapies, Pozelimab appears to be the drug that can potentially transform the Paroxysmal Nocturnal Hemoglobinuria Drugs Market.
Further details are provided in the report…
Latest KOL Views
To stay abreast of the latest trends in the market, we conduct primary research by seeking the opinions of Key Opinion Leaders (KOLs) and Subject Matter Experts (SMEs) who work in the relevant field. This helps us fill any gaps in data and validate our secondary research.
We have reached out to industry experts to gather insights on various aspects of Paroxysmal Nocturnal Hemoglobinuria, including the evolving treatment landscape, patients’ reliance on conventional therapies, their acceptance of therapy switching, drug uptake, and challenges related to accessibility. The experts we contacted included medical/scientific writers, professors, and researchers from prestigious universities in the US, Europe, the UK, and Japan.
Our team of analysts at Delveinsight connected with more than 15 KOLs across the 7MM. We contacted institutions such as the University of Tsukuba, Duke University, University of Glasgow, Washington University School of Medicine, etc., among others. By obtaining the opinions of these experts, we gained a better understanding of the current and emerging treatment patterns in the Paroxysmal Nocturnal Hemoglobinuria market, which will assist our clients in analyzing the overall epidemiology and market scenario.
Paroxysmal Nocturnal Hemoglobinuria Qualitative Analysis
We perform Qualitative and Market Intelligence analysis using various approaches, such as SWOT analysis and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the Analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving Paroxysmal Nocturnal Hemoglobinuria Treatment Market Landscape.
Conjoint Analysis analyzes multiple approved and Paroxysmal Nocturnal Hemoglobinuria emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, designation, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy. In efficacy, the trial’s primary and secondary outcome measures are evaluated; for instance, in trials for Paroxysmal Nocturnal Hemoglobinuria, one of the most important primary endpoints was achieving LDH percentage change, transfusion avoidance, etc. Based on these, the overall efficacy is evaluated.
Further, the therapies’ safety is evaluated, wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the route of administration, order of entry and designation, probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Paroxysmal Nocturnal Hemoglobinuria Market Access and Reimbursement
Because newly authorized Paroxysmal Nocturnal Hemoglobinuria drugs are often expensive, some patients escape receiving proper treatment or use off-label, less expensive prescriptions. Reimbursement plays a critical role in how innovative treatments can enter the market. The cost of the medicine, compared to the benefit it provides to patients who are being treated, sometimes determines whether or not it will be reimbursed. Regulatory status, target population size, the setting of treatment, unmet needs, the number of incremental benefit claims, and prices can all affect market access and reimbursement possibilities.
The Paroxysmal Nocturnal Hemoglobinuria Therapeutics Market Report further provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of approved therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Paroxysmal Nocturnal Hemoglobinuria Market Report Scope
- The Paroxysmal Nocturnal Hemoglobinuria Treatment Market Report offers extensive knowledge regarding the epidemiology segments and predictions, presenting a deep understanding of the potential future growth in diagnosis rates, disease progression, and treatment guidelines. It provides comprehensive insights into these aspects, enabling a thorough assessment of the subject matter.
- Additionally, an all-inclusive account of the current management techniques and emerging therapies and the elaborative profiles of late-stage (Phase III and Phase II) and prominent therapies that would impact the current Paroxysmal Nocturnal Hemoglobinuria Treatment Market Landscape and result in an overall market shift has been provided in the report.
- The Paroxysmal Nocturnal Hemoglobinuria Treatment Market Report also encompasses a comprehensive analysis of the market, providing an in-depth examination of its historical and projected market size (2020–2034). It also includes the Paroxysmal Nocturnal Hemoglobinuria Drugs Market Share of therapies, detailed assumptions, and the underlying rationale for our methodology. The report also includes drug outreach coverage in the 7MM region.
- The Paroxysmal Nocturnal Hemoglobinuria Treatment Market Report includes qualitative insights that provide an edge while developing business strategies by understanding trends through SWOT analysis and expert insights/KOL views, including experts from various hospitals and prominent universities, patient journey, and treatment preferences that help shape and drive the 7MM Paroxysmal Nocturnal Hemoglobinuria Drugs Market.
Paroxysmal Nocturnal Hemoglobinuria Therapeutics Market Report Insights
- Patient-based Paroxysmal Nocturnal Hemoglobinuria Market Forecasting
- Therapeutic Approaches
- Paroxysmal Nocturnal Hemoglobinuria Market Size and Trends
- Existing Paroxysmal Nocturnal Hemoglobinuria Drugs Market Opportunity
Paroxysmal Nocturnal Hemoglobinuria Therapeutics Market Report Key Strengths
- 10-year Paroxysmal Nocturnal Hemoglobinuria Market Forecast
- The 7MM Coverage
- Paroxysmal Nocturnal Hemoglobinuria Epidemiology Segmentation
- Key Cross Competition
Paroxysmal Nocturnal Hemoglobinuria Therapeutics Market Report Assessment
- Current Paroxysmal Nocturnal Hemoglobinuria Treatment Practices
- Reimbursements
- Paroxysmal Nocturnal Hemoglobinuria Drugs Market Attractiveness
- Qualitative Analysis (SWOT, Conjoint Analysis, Unmet Needs)
Key Questions answered through our Paroxysmal Nocturnal Hemoglobinuria Market Report:
- Would there be any changes observed in the current treatment approach?
- Will there be any improvements in Paroxysmal Nocturnal Hemoglobinuria management recommendations?
- Would research and development advances pave the way for future tests and therapies for Paroxysmal Nocturnal Hemoglobinuria?
- Would the diagnostic testing space experience a significant impact and lead to a positive shift in the treatment landscape of Paroxysmal Nocturnal Hemoglobinuria?
- What kind of uptake will the new therapies witness in the coming years in Paroxysmal Nocturnal Hemoglobinuria patients?
Stay updated with us for Recent Articles