Persistent Epithelial Defects Market
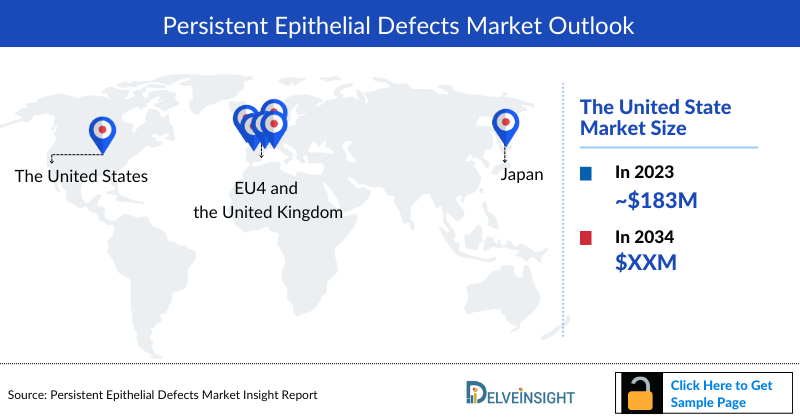
- In 2023, the Persistent Epithelial Defects Market Size was highest in the US among the 7MM, accounting for ~USD 183 million, which is further expected to increase by 2034.
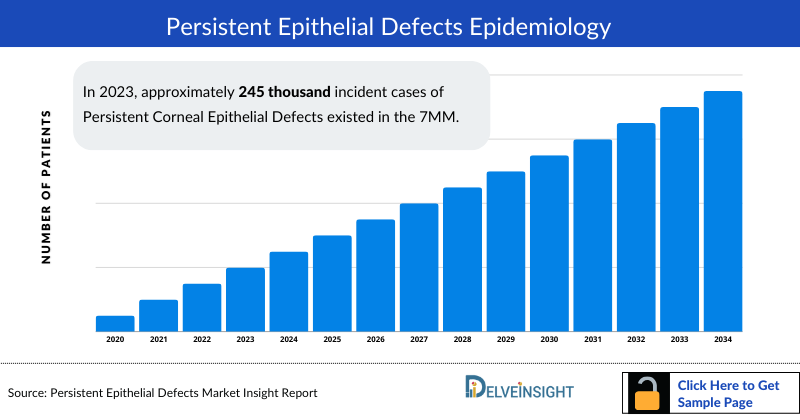
- In 2023, approximately 2,45,000 Persistent Epithelial Defects Incident Cases existed in the 7MM. Factors contributing to the rise of incident cases of Persistent Epithelial Defects in the 7MM are attributed to the aging population, ocular surface disorders, neurological conditions, and environmental factors.
- In Gender-specific instances of Persistent Epithelial Defects in the US, a higher number of cases were observed in males compared to females, comprising approximately 63 thousand cases in 2023.
- The ePersistent Epithelial Defects Emerging Drug NEXAGON is expected to launch in Japan by 2026, which has the potential to reduce the disease burden of Persistent Epithelial Defects in the forecasted years.
Request for Unlocking the Sample Page of the "Persistent Epithelial Defects Treatment Market"
DelveInsight's “Persistent Epithelial Defects Treatment Market Insights, Epidemiology, and Market Forecast – 2034” report delivers an in-depth understanding of the Persistent Epithelial Defects, historical and forecasted epidemiology as well as the Persistent Epithelial Defects market trends in the United States, EU4 (Germany, Spain, Italy, and France) and the United Kingdom, and Japan.
The Persistent Epithelial Defects Treatment Market Report provides current treatment practices, emerging drugs, and market share of the individual therapies, current and forecasted 7MM Persistent Epithelial Defects market size from 2020 to 2034. The report also covers current Persistent Epithelial Defects Treatment Market practices/algorithms and unmet medical needs to curate the best of the opportunities and assess the underlying potential of the Persistent Epithelial Defects drugs market.
|
Study Period |
2020 to 2034 |
|
Forecast Period |
2024-2034 |
|
Geographies Covered |
|
|
Persistent Epithelial Defects Market |
|
|
Persistent Epithelial Defects Market Size |
USD 274 Million in 2023 |
|
Persistent Epithelial Defects Companies |
|
Persistent Epithelial Defects Treatment Market
Persistent Epithelial Defects represents a challenging ophthalmic condition characterized by the failure of the corneal epithelium to heal within the expected timeframe, typically persisting for more than two weeks. Symptomatically, patients with Persistent Epithelial Defects often experience chronic pain, foreign body sensation, photophobia, blurred vision, and tearing. Etiologically, Persistent Epithelial Defects arises from a disruption in the normal healing process of the corneal epithelium, which can be attributed to a variety of underlying causes. These include trauma, surgical interventions, and infections (bacterial, viral, or fungal). Risk factors encompass a range of chronic conditions such as diabetes mellitus, dry eye syndrome, and neurotrophic keratopathy. Additionally, the use of contact lenses, prolonged exposure to topical medications containing preservatives, and environmental factors like UV radiation and pollutants exacerbate the condition. The multifactorial nature of Persistent Epithelial Defects necessitates a comprehensive understanding of its pathophysiology to tailor effective therapeutic strategies and improve patient outcomes.
Persistent Epithelial Defects Diagnosis
Diagnosing Persistent Epithelial Defects involves fluorescein instillation to assess defect size, location, and depth, with deeper PEDs showing prolonged absorption. A thorough physical exam should reveal anterior chamber inflammation, eyelid abnormalities, or reduced corneal sensation, indicative of neurotrophic PED. Distinguishing PED from a simple epithelial defect relies on the defect's persistence beyond two weeks. Detailed patient history, including prior herpetic infections, diabetes, and immune disorders, is crucial. Rare conditions like limbal stem cell deficiency (LSCD) may present with scarring and neovascularization. Persistent Epithelial Defectss, associated with various ocular and systemic diseases, require identifying underlying autoimmune disorders for effective management. Diagnosis includes clinical history, facial and eyelid exams, corneal sensation testing, and evaluation of epithelial defect characteristics. Excluding systemic and local causes, including neurological issues, often necessitates collaboration with a neurologist and cranial imaging. Corneal sensitivity and Schirmer tests, along with vital staining, aid in assessment, while microbiological exams rule out infections.
Further details related to diagnosis are provided in the report…
Persistent Epithelial Defects Treatment
Persistent Epithelial Defects Treatment requires a multifaceted approach to enhance corneal healing and prevent complications. Lubrication with preservative-free artificial tears and ointments maintains moisture and promotes epithelialization. Bandage soft contact lenses serve as a protective barrier, reducing friction and facilitating healing. Punctal plugs, which occlude the tear ducts, increase tear film stability and moisture retention on the ocular surface. Debridement involves the careful removal of non-viable epithelial cells to stimulate the regrowth of healthy tissue. For severe or refractory cases, tarsorrhaphy, a surgical procedure that partially or completely sews the eyelids together, may be employed to protect the cornea and reduce exposure. This comprehensive management strategy, tailored to the patient's specific needs, aims to restore corneal integrity and improve visual outcomes.
Persistent Epithelial Defects Epidemiology
As the market is derived using a patient-based model, the Persistent Epithelial Defects epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by Incident Cases of Persistent Corneal Epithelial Defects, Incident cases of Persistent Corneal Epithelial Defects based on Gender, Etiology-specific Incidence of Persistent Corneal Epithelial Defects in the 7MM covering, the United States, EU4 countries (Germany, France, Italy, and Spain), United Kingdom, and Japan from 2020 to 2034.
- The Persistent Epithelial Defects Incident Cases were highest in the US among the 7MM in 2023, with approximately 105 thousand cases. These cases are anticipated to grow in the foreseeable future during the study period.
- The categorization based on gender, showed that incident cases in males were higher than that in females in Japan. The male Persistent Epithelial Defects Incident Cases accounted for 60% of the total cases in Japan.
- The Persistent Epithelial Defects Etiology-specific Incident Population was divided into cases caused by other inflammatory diseases, neurotrophic keratitis, epithelial/limbal stem cell deficiency, and others (including mechanical, idiopathic, etc.) cases of Persistent Epithelial Defects. Among the EU4 and the UK, Germany had the highest number of etiology-specific incident cases of Persistent Epithelial Defects, with other inflammatory diseases accounting for the highest cases, approximately 11 thousand, in 2023.
Persistent Epithelial Defects Drugs Market Chapters
The drug chapter segment of the Persistent Epithelial Defects therapeutics market report encloses a detailed analysis of Persistent Epithelial Defects off-label drugs and late-stage (Phase-III and Phase-II) Persistent Epithelial Defects pipeline drugs analysis. It also helps to understand the Persistent Epithelial Defects clinical trials details, expressive pharmacological action, agreements and collaborations, approval and patent details, advantages and disadvantages of each included drug, and the latest Persistent Epithelial Defects news and press releases.
Persistent Epithelial Defects Marketed Drugs
- OXERVATE: Dompe Farmaceutici
OXERVATE (cenegermin-bkbj) ophthalmic solution 0.002%, developed by Dompe Farmaceutici, treats Stage 2 (persistent epithelial defect) and Stage 3 (corneal ulcer) neurotrophic keratitis. Cenegermin, a recombinant human nerve growth factor (rhNGF) produced in Escherichia coli, discovered by Nobel Laureate Rita Levi Montalcini, supports corneal innervation and integrity through high-affinity TrkA and low-affinity p75NTR receptors. It promotes corneal epithelial cell growth and survival, maintaining limbal epithelial stem cell potential, crucial for overcoming neurotrophic keratitis. OXERVATE, the first topical biologic medication approved in ophthalmology and the first rhNGF application, received EMA (2017) and FDA (2018) approval. It is available in the US and Europe, excluding the UK due to NICE's cost concerns, and is a first-line treatment for Stage 2 or 3 neurotrophic keratitis patients.
Persistent Epithelial Defects Emerging Drugs
- NEXAGON (Lufepirsen ophthalmic gel): Amber Ophthalmics
NEXAGON, a first-in-class natural antisense oligodeoxynucleotide (30-base), is under development by Amber Ophthalmics for the treatment of Persistent Epithelial Defects. It targets connexin43 (Cx43), a protein involved in pathological hemichannel formation and inflammation. By inhibiting Cx43 overexpression, NEXAGON disrupts the inflammatory cascade and supports corneal epithelial regeneration. Formulated as a thermoreversible gel, NEXAGON is applied under a contact lens or amniotic membrane to ensure prolonged contact with the corneal surface. The drug has received orphan drug designation from the FDA and is currently in Phase II Plaque Modification Devices Clinical Trials.
- KPI-012: Kala Bio
KPI-012 is a topically dosed biologic therapy initially being developed, with FDA orphan drug and fast track designations, for the treatment of persistent corneal epithelial defect (Persistent Epithelial Defects). Extensively characterized, KPI-012 contains dozens of human proteins known to facilitate normal corneal tissue repair, including key classes of corneal repair proteins such as protease inhibitors, matrix proteins, and growth factors. KPI-012 has the potential to be the first approved treatment for Persistent Epithelial Defects with a broad indication and a differentiated product profile, potentially providing rapid and sustained wound healing, easy self-administration, and a mechanism of action that can address all etiologies. Currently, KPI-012 is in Phase IIb development for Persistent Epithelial Defects in the CHASE (Corneal Healing After Secretome Therapy) Phase IIb trial.
|
Drug |
MoA |
RoA |
Company |
Logo |
Phase |
|
Nexagon |
Cx43 inhibitors; Gap junction modulators |
Topical |
Amber Ophthalmics |
II | |
|
KPI-012 |
Collagen replacements; protease inhibitors |
Topical |
Kala Bio |
|
IIb |
|
XXX |
XXX |
Topical |
XXX |
XXX |
II |
Persistent Epithelial Defects Market Outlook
Managing Persistent Epithelial Defects is complex due to diverse underlying causes and healing challenges. Conventional treatments include aggressive lubrication with preservative-free artificial tears or ophthalmic ointments, punctal occlusion, bandage soft contact lenses, or pressure patching. Advanced options for refractory cases involve amniotic membrane grafting, autologous serum drops, and scleral contact lenses.
Amniotic membrane grafting utilizes the regenerative properties of the human amniotic membrane, available as direct corneal applications or within PMMA rings (e.g., Prokera). Autologous serum drops provide therapeutic benefits with growth factors but are limited by cost and procedural complexity. Emerging alternatives like umbilical cord blood serum (CBS) tears and platelet-rich fibrin (PRF) tears show promise, with CBS offering higher growth factor levels.
Limbal stem cell transplantation is recommended for underlying deficiencies, such as in ocular cicatricial pemphigoid or Stevens-Johnson syndrome. Scleral and PROSE lenses also aid in managing Persistent Epithelial Defects. Keeping in mind, that the current strategies are insufficient to manage the disease burden there is an urgent need for the development of novel therapy. The market awaits the launch of the potential entities NEXAGON, KPI-012, and others that would be a helpful in the reduction of affected populations accomplishing a critical global public health need, if proven to be efficacious.
- The total Persistent Epithelial Defects Market Size in the 7MM was ~USD 274 million in 2023 and is projected to increase during the forecast period (2024–2034).
- The Persistent Epithelial Defects Market Size for the US is the highest comprising approximately 66% of the total Persistent Epithelial Defects Market Size in the 7MM in 2023.
- Among EU countries, Germany accounted for the maximum market size of USD 18 million in 2023 while the UK occupied the bottom of the ladder in the same year with USD 8 million.
- In 2023, Japan accounted for 8% of the total Persistent Epithelial Defects Market Size in the 7MM, amounting to USD 22 million.
Persistent Epithelial Defects Drugs Uptake
This section focuses on the uptake rate of potential Persistent Epithelial Defects drugs expected to launch in the market during 2020–2034. For example, NEXAGON in the EU4 and the UK is expected to be launched by 2025 with a peak shared of 10%. NEXAGON is anticipated to take 7 years to peak with a medium uptake.
Persistent Epithelial Defects Pipeline Development Activities
The Persistent Epithelial Defects therapeutics market report provides insights into different therapeutic candidates in Phase III, Phase II, and Phase I. It also analyzes key Persistent Epithelial Defects Companies involved in developing targeted therapeutics.
Pipeline Development Activities
The report covers information on collaborations, acquisitions and mergers, licensing, and patent details for Persistent Epithelial Defects emerging therapies.
KOL Views
To keep up with current market trends, we take KOLs and SMEs’ opinions working in the domain through primary research to fill the data gaps and validate the secondary research. Industry Experts were contacted for insights on Persistent Epithelial Defects evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake along with challenges related to accessibility, including KOL from the University of Texas, Houston, TX, USA; Department of Ophthalmology, Shizuoka, Japan; Clinician at BostonSight, US; Hospital La Princesa; Department of Ophthalmology and Visual Sciences, University of Maryland School of Medicine, Moorfields Eye Hospital, London, and others.
Delveinsight’s analysts connected with 50+ KOLs to gather insights; however, interviews were conducted with 15+ KOLs in the 7MM. Their opinion helps understand and validate current and emerging therapies, treatment patterns, or Persistent Epithelial Defects market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
Persistent Epithelial Defects Drugs Market: Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the Analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving treatment landscape.
Conjoint Analysis analyzes multiple approved and emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy.
Further, the therapies’ safety is evaluated wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the route of administration, order of entry and designation, probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Persistent Epithelial Defects Therapeutics Market Access and Reimbursement
The high cost of therapies for the treatment is a major factor restraining the growth of the global drug market. Because of the high cost, the economic burden is increasing, leading the patient to escape from proper treatment. The Persistent Epithelial Defects therapeutics market report further provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of approved therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Persistent Epithelial Defects Therapeutics Market Report Scope
- The Persistent Epithelial Defects Therapeutics Market Report covers a segment of key events, an executive summary, and a descriptive overview of Persistent Epithelial Defects, explaining its causes, signs, and symptoms, and currently available therapies.
- Comprehensive insight has been provided into the epidemiology segments and forecasts, the future growth potential of diagnosis rate, disease progression, and treatment guidelines.
- Additionally, an all-inclusive account of the current and emerging therapies and the elaborate profiles of late-stage and prominent therapies will impact the current Persistent Epithelial Defects Treatment Market Landscape.
- A detailed review of the Persistent Epithelial Defects Treatment Market, historical and forecasted Persistent Epithelial Defects Market Size, Persistent Epithelial Defects Drugs Market Share by therapies, detailed assumptions, and rationale behind the approach is included in the report covering the 7MM drug outreach.
- The Persistent Epithelial Defects Therapeutics Market Report provides an edge while developing business strategies, by understanding trends, through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help shape and drive the 7MM Persistent Epithelial Defects Drugs Market.
Persistent Epithelial Defects Therapeutics Market Report Insights
- Patient-based Persistent Epithelial Defects Market Forecasting
- Persistent Epithelial Defects Therapeutics Approaches
- Persistent Epithelial Defects Pipeline Drugs Analysis
- Persistent Epithelial Defects Market Size and Trends
- Existing and Future Persistent Epithelial Defects Drugs Market Opportunity
Persistent Epithelial Defects Therapeutics Market Report Key Strengths
- 11 years Persistent Epithelial Defects Market Forecast
- The 7MM Coverage
- Persistent Epithelial Defects Epidemiology Segmentation
- Key Cross Competition
- Conjoint Analysis
- Persistent Epithelial Defects Drugs Uptake
- Key Persistent Epithelial Defects Market Forecast Assumptions
Persistent Epithelial Defects Therapeutics Market Report Assessment
- Current Persistent Epithelial Defects Treatment Market Practices
- Persistent Epithelial Defects Unmet Needs
- Persistent Epithelial Defects Pipelined Drugs Analysis Profiles
- Persistent Epithelial Defects Drugs Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
Key Questions
Persistent Epithelial Defects Treatment Market Insights
- What was the Persistent Epithelial Defects Market Size, the Persistent Epithelial Defects treatment market size by therapies, and Persistent Epithelial Defects drugs market share (%) distribution in 2020, and how it would all look in 2034? What are the contributing factors for this growth?
- Will the coverage of drugs depend on their efficacy in Persistent Epithelial Defects?
- What will be the impact on the market with the launch of emerging drugs?
- How is NEXAGON going to compete with KPI-012 being launched in the same year?
- Which is going to be the largest contributor to the market in 2034?
- What are the pricing variations among different geographies for approved and off-label therapies?
- How would the market drivers, barriers, and future opportunities affect the market dynamics and subsequent analysis of the associated trends?
Persistent Epithelial Defects Epidemiology Insights
- What are the disease risks, burdens, and Persistent Epithelial Defects Unmet Needs? What will be the growth opportunities across the 7MM with respect to the patient population pertaining to Persistent Epithelial Defects?
- What is the historical and forecasted Persistent Epithelial Defects patient pool in the United States, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan?
- Why do patients develop persistent Persistent Epithelial Defects symptoms? Why is the current year diagnosis rate not high?
- Which type of severity is the largest contributor in patients affected with Persistent Epithelial Defects?
- What factors are affecting the increase in the Persistent Epithelial Defects Diagnosis?
Current Persistent Epithelial Defects Treatment Market Scenario, Marketed Drugs, and Emerging Therapies
- What are the current options for the Persistent Epithelial Defects Treatment? What are the current treatment guidelines for the treatment of Persistent Epithelial Defects in the US and Europe?
- How many companies are developing therapies for the treatment of Persistent Epithelial Defects?
- How, many emerging therapies are in the mid-stage and late stage of development for the treatment of Persistent Epithelial Defects?
- What are the recent novel therapies, targets, mechanisms of action, and technologies developed to overcome the limitations of existing therapies?
- What are the key designations that have been granted for the emerging therapies for Persistent Epithelial Defects?
- What will be the impact on the market after the expected patent expiry of the emerging drug?
- What is the cost burden of off-label therapies on patients?
- Patient acceptability in terms of preferred treatment options as per real-world scenarios?
- What are the country-specific accessibility issues of recommended therapies? Focus on reimbursement policies.
- What are the 7MM historical and forecasted Persistent Epithelial Defects Drugs Market?
Reasons to Buy
- The Persistent Epithelial Defects Therapeutics Market Report will help in developing business strategies by understanding the latest trends and changing treatment dynamics driving the Persistent Epithelial Defects Drugs Market.
- Insights on patient burden/disease Persistent Epithelial Defects Incidence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years.
- To understand the existing Persistent Epithelial Defects Drugs Market opportunity in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
- Identification of strong upcoming players in the Persistent Epithelial Defects Drugs Market will help in devising strategies that will help in getting ahead of competitors.
- Detailed analysis and potential of current and emerging therapies under the conjoint analysis section to provide visibility around leading emerging drugs.
- Highlights of Access and Reimbursement policies of approved therapies, barriers to accessibility of off-label expensive therapies, and patient assistance programs.
- To understand the perspective of Key Opinion Leaders around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing Persistent Epithelial Defects Drugs Market so that the upcoming players can strengthen their development and launch strategy.
Stay Updated with us for Recent Articles @ Latest DelveInsight Blogs


-market.png&w=256&q=75)

