Pheochromocytomas and Paragangliomas Market Summary
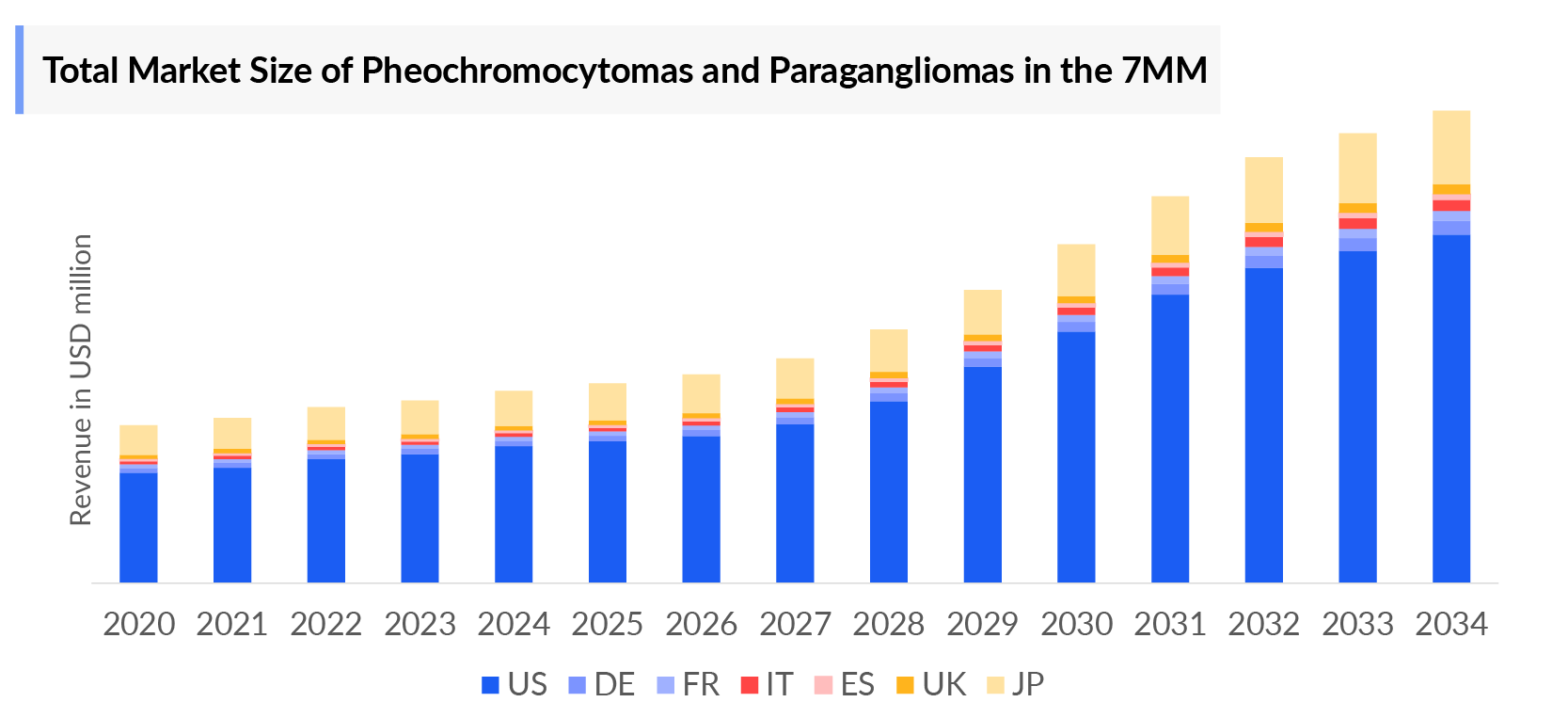
- The Pheochromocytomas and Paragangliomas market size in the 7MM is expected to grow from USD 314 million in 2025 to USD 662 million in 2034.
- The Pheochromocytomas and Paragangliomas market is projected to grow at a CAGR of 8.60% by 2034 in leading countries like US, EU4, UK and Japan.
Pheochromocytomas and Paragangliomas Market and Epidemiology Analysis
- For functioning metastatic PCPG, it is recommended to start with alpha blockade before therapy, followed by beta-blockers, calcium channel blockers, and metyrosine as needed.
- Radical surgical resection is the cornerstone of the treatment for the majority of localized PPGLs as it remains the only potentially curative therapeutic option.
- The metastatic Pheochromocytomas and Paragangliomas treatment market has experienced both advancements and challenges. While curative options remain unavailable, patients are managed with a multidisciplinary approach, including debulking surgery, chemotherapy (such as the cyclophosphamide, vincristine, and dacarbazine [CVD] regimen with cyclophosphamide, dacarbazine, and vincristine), tyrosine kinase inhibitors, immunotherapies, and radionuclide therapy with 177Lu-DOTATATE.
- According to international guides and overseas clinical practice guidelines, combination therapy of CVD therapy is one choice for the pheochromocytoma treatment. However, CVD therapy was not covered by the Japanese national health insurance system for the pheochromocytoma treatment.
- The AZEDRA withdrawal from the market due to commercial non-viability highlights the challenges associated with drug development for ultra-rare diseases such as PCPG. AZEDRA (HSA 131I-MIBG) was first approved in 2018 by the FDA, but in 2023, the company decided to discontinue the drug. The company continued the manufacturing till Q1 2024.
- An alternative first-line approach for cluster 1 SDHx-related PCPG is PRRT, which uses SSAs labeled with isotopes to deliver cytotoxic radionuclides. PRRT is currently being explored for PCPG, with 177Lu-DOTATATE (LUTATHERA) under investigation in clinical trials.
- In January 2025, the US FDA accepted for Priority Review a sNDA seeking approval of WELIREG for the treatment of adult and pediatric patients (12 years and older) with advanced, unresectable, or metastatic PCPG. The FDA has set a PDUFA, or target action, date of May 26, 2025
- In November 2024, Perspective Therapeutics presented the initial results from its ongoing Phase I/IIa clinical trial of [212Pb] VMT-a-NET at the 2024 NANETS Multidisciplinary NET Medical Symposium, held from November 21 to 23 in Chicago.
- With the Prescription Drug User Fee Act date of May 2025, Merck's WELIREG (Belzutifan/MK-6482) is poised for launch, offering a promising treatment option for adults and pediatric patients aged 12 years and older with advanced PCPG.
- Emerging companies such as Chimerix, Merck, Novartis, Perspective Therapeutics, and others are evaluating their Pheochromocytomas and Paragangliomas therapies.
Pheochromocytomas and Paragangliomas Market size and forecast:
- 2025 Market Size: USD 314 million in 2025
- 2034 Projected Market Size: USD 662 million in 2034
- Growth Rate (2025-2032): 8.60% CAGR
- Largest Market: United States
Key Factors Driving the Pheochromocytomas & Paragangliomas (PCPG) Market
PCPG incidence and emerging demand
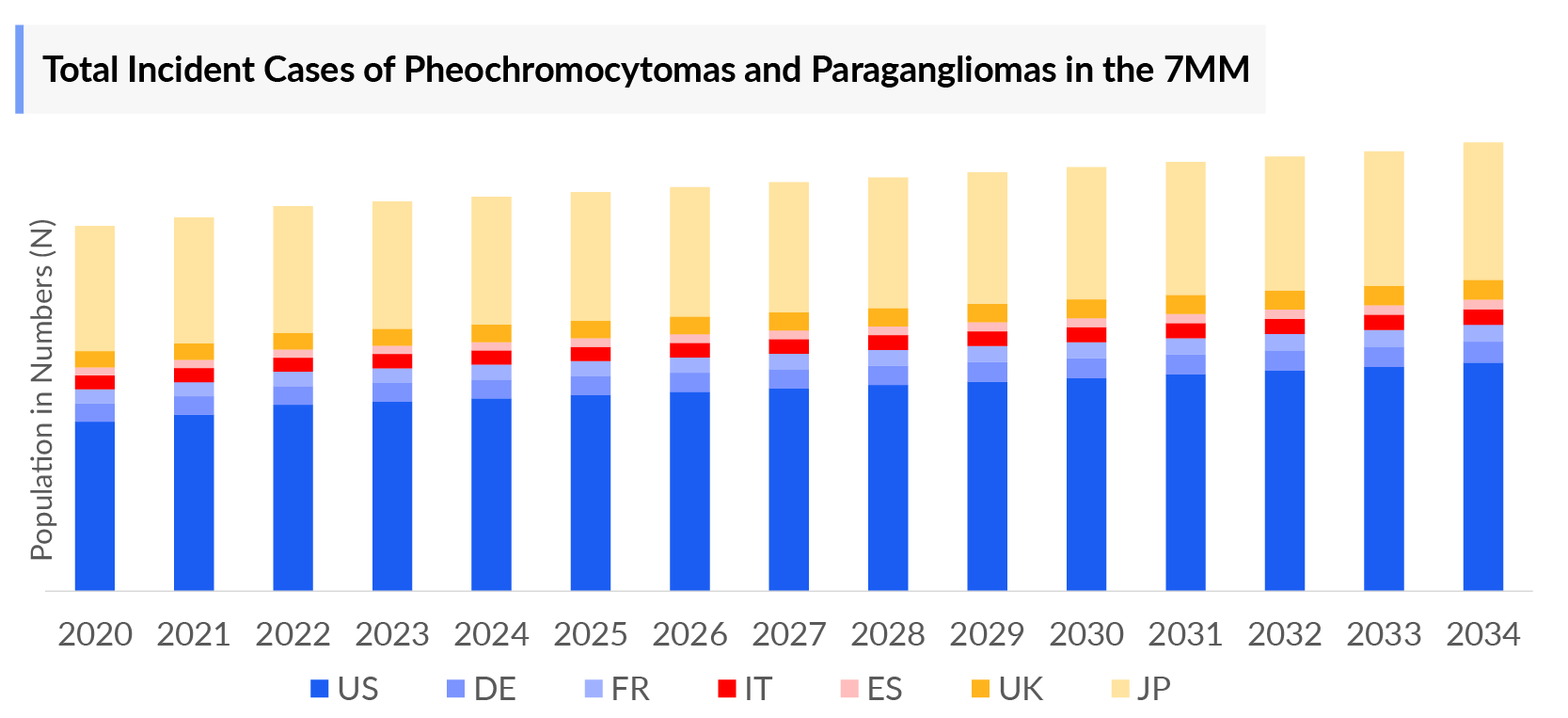
The total number of Pheochromocytomas and Paragangliomas incident cases in the 7MM was nearly 5K in 2024 and is projected to rise by 2034. Growing adoption of advanced imaging, wider genetic screening, and increasing clinician awareness are expected to expand the PCPG market by improving diagnosis rates and fueling demand for systemic PCPG treatments.
Pheochromocytomas & Paragangliomas Current treatment landscape
Surgery is the mainstay for localized PCPG, while systemic options remain scarce. DEMSER (metyrosine, 1979, US) is still used in select cases, and AZEDRA (iobenguane I 131, Lantheus) gained FDA approval in 2018 but was discontinued in 2023 due to low demand. A breakthrough came in May 2025 with FDA approval of WELIREG (belzutifan, Merck), the first new systemic therapy in years, offering fresh hope for advanced PCPG treatment and reshaping the PCPG market.
Pheochromocytomas & Paragangliomas Clinical Trial Activity
The PCPG pipeline features genotype-driven TKIs, radioligand therapies, and novel targeted constructs, with momentum led by biotech innovators and academic consortia. Notable candidates include ONC201 (Chimerix), WELIREG (Merck), LUTATHERA (Novartis), and VMT-α-NET (Perspective Therapeutics), alongside other radiopharmaceutical and immunotherapy approaches. These agents aim to address the long-standing treatment gap in advanced PCPG, signaling a more competitive and diversified future market.
Pheochromocytomas & Paragangliomas Market Implications
For a small, high-need patient cohort, the PCPG market offers strategic appeal, success for novel treatments hinges on genetic or receptor-targeted efficacy, CNS penetration, and payer acceptance of orphan-indication pricing.
DelveInsight’s “Pheochromocytomas and Paragangliomas Market Insights, Epidemiology, and Market Forecast 2034” report delivers an in-depth understanding of PCPG, historical and forecasted epidemiology as well as Pheochromocytomas and Paragangliomas therapeutics market trends in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
The Paragangliomas and Pheochromocytomas market report provides current treatment practices, emerging drugs, market share of individual therapies, and current and forecasted 7MM Pheochromocytomas and Paragangliomas market size from 2020 to 2034. The report also covers current PCPG treatment practices/algorithms and unmet medical needs to curate the best opportunities and assess the Pheochromocytomas and Paragangliomas market potential.
| Scope of the Paragangliomas and Pheochromocytomas market report | |
|
Study Period |
2020 to 2034 |
|
Forecast Period |
2025-2034 |
|
Geographies Covered |
|
|
Pheochromocytomas and Paragangliomas Market |
|
|
Pheochromocytomas and Paragangliomass Market Size | |
|
Pheochromocytomas and Paragangliomas Companies |
Chimerix, Ohara Pharmaceutical, Merck, Novartis, Perspective Therapeutics, and others. |
|
Pheochromocytomas and Paragangliomas Epidemiology Segmentation |
|
Pheochromocytomas and Paragangliomas Disease Understanding
Pheochromocytomas and Paragangliomas Overview
Pheochromocytomas and paragangliomas are highly vascular neuroendocrine tumors that arise from chromaffin cells of the adrenal medulla or their neural crest progenitors located outside of the adrenal gland, respectively. These neuroendocrine tumors are classified as tumors of the adrenal medulla and extra-adrenal paraganglia. These tumors are derived either from sympathetic tissue in adrenal or extra-adrenal abdominal locations (sympathetic) or from parasympathetic tissue in the thorax or head and neck (parasympathetic). Sympathetic ones frequently produce considerable amounts of catecholamines, and in approximately 80% of patients, they are found in the adrenal medulla. The remaining 20% of these tumors are located outside of the adrenal glands, in the prevertebral and paravertebral sympathetic ganglia of the chest, abdomen, and pelvis. Extra-adrenal para in the abdomen most commonly arises from a collection of chromaffin tissue around the origin of the inferior mesenteric artery (the organ of Zuckerkandl) or aortic bifurcation. In contrast, most parasympathetic ones are chromaffin-negative tumors mostly confined to the neck and at the base of the skull region along the glossopharyngeal and vagal nerves, and only 4% of these tumors secrete catecholamines.
Pheochromocytomas and Paragangliomas Diagnosis
The PCPG diagnosis relies on confirming catecholamine excess through biochemical testing and localizing the tumor via imaging. Biochemical testing is crucial, with metanephrines and normetanephrine, metabolites of epinephrine and norepinephrine, being more reliable markers than circulating catecholamines. Genetic testing is recommended for all pheochromocytoma and paraganglioma patients, as it can impact surgical planning. Next-generation sequencing (NGS) has become the preferred method due to its efficiency and ability to test for common predisposing genes such as SDHB, VHL, and RET. The pheochromocytoma diagnosis and treatment market is evolving with advanced technologies, innovative therapies, and increasing awareness for better management.
Following biochemical confirmation, radiologic evaluation using CT and MRI is essential for planning surgery. CT is often the initial imaging method, particularly for small adrenal tumors, while MRI may be more appropriate for head and neck tumors. Functional imaging, like 123I-MIBG, is typically reserved for assessing metastatic PCPG in patients who might be candidates for 131I-MIBG therapy, though its routine use is not recommended due to the potential for misleading results. This comprehensive approach ensures accurate diagnosis and effective treatment planning for pheochromocytoma and paraganglioma patients.
Further details related to diagnosis are provided in the report…
Pheochromocytomas and Paragangliomas Treatment
Current metastatic Pheochromocytomas and Paragangliomas treatments are primarily palliative, with surgery offering potential curative outcomes only when tumor spread is limited. Other treatment options include cytoreductive techniques, chemotherapy, radiotherapy, and experimental therapies. Targeted therapies are tailored to specific genetic clusters of PCPG. The radionuclide injection market forecast predicts significant growth driven by advancements in nuclear medicine, increasing diagnostic applications, and rising healthcare investments.
In the pseudohypoxia group (cluster 1), therapies include antiangiogenic treatments, HIF inhibitors, immunotherapy, and PARP inhibitors. Cluster 2, or the kinase signaling group, may benefit from mTOR inhibitors like everolimus. Although there are no specific therapies for the Wnt signaling group (Cluster 3), other non-cluster-specific treatments show promise. These include MIBG, combination chemotherapy with CVD, peptide receptor radionucleotide therapy (PRRT) targeting somatostatin receptors, and the chemotherapy drug temozolomide (TMZ), an oral alternative to dacarbazine.
Further details related to treatment are provided in the report…
Pheochromocytomas and Paragangliomas Epidemiology
The Pheochromocytomas and Paragangliomas epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by Pheochromocytomas and Paragangliomas Incident Cases, Occurrence or Absence of Mutation in PCPG, Age-specific Cases of PCPG, and Stage-specific Cases of PCPG in the 7MM covering the United States, EU4 (Germany, France, Italy, and Spain), United Kingdom, and Japan from 2020 to 2034.
Key Findings from Pheochromocytomas and Paragangliomas Epidemiological Analysis and Forecast:
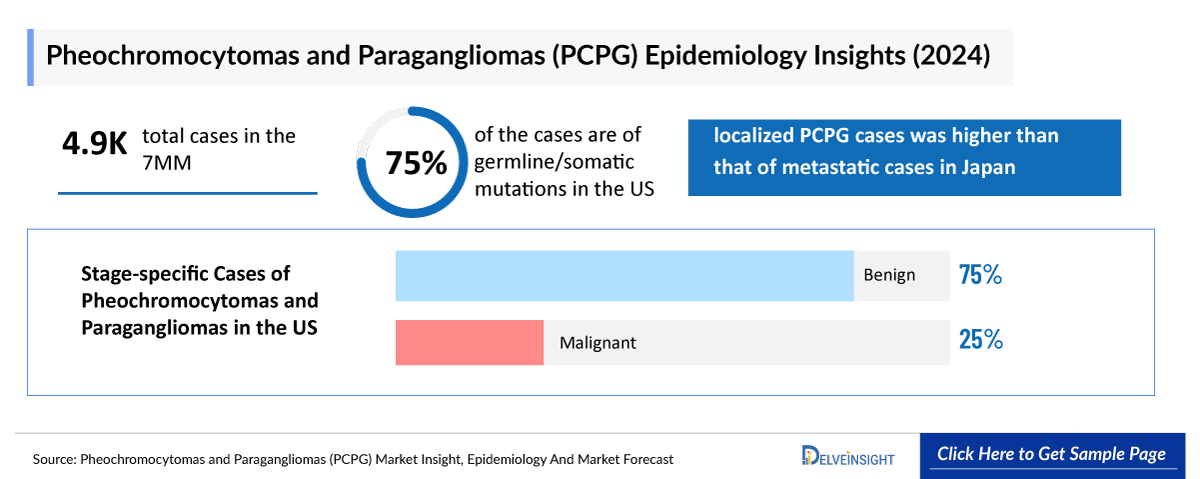
- The total number of Pheochromocytomas and Paragangliomas incident cases in the 7MM was nearly 4,900 cases in 2024 and is projected to increase during the forecasted period.
- In 2024, it has been observed that germline/somatic mutations are present in nearly 75% of all the cases in the US.
- The total number of PCPG incident cases in EU4 and the UK was estimated to be nearly 1,800 cases in 2024.
- Among EU4 and the UK, Germany accounted for the highest number of incident cases of pheochromocytoma and paraganglioma, while Spain had the least number of cases, in 2024.
- Among the stage-specific cases, the number of localized PCPG cases was higher than that of metastatic cases in Japan.
Pheochromocytomas and Paragangliomas Epidemiology Segmentation:
- Pheochromocytomas and Paragangliomas Incident Cases
- Occurrence or Absence of Mutation in PCPG
- Age-specific Cases of PCPG
- Stage-specific Cases of PCPG
Pheochromocytomas and Paragangliomas Market Recent Developments and Breakthroughs
- In May 2025, the FDA approved Merck’s Welireg (belzutifan) as the first oral treatment for advanced pheochromocytoma and paraganglioma (PPGL) in patients aged 12 and older.
Pheochromocytomas and Paragangliomas Drug Chapter Analysis
The drug chapter segment of the Pheochromocytomas and Paragangliomas report encloses a detailed analysis of the marketed and the mid and early stage (Phase II, Phase I/II and Phase I) pipeline drug. The marketed drugs segment encloses drugs such as DEMSER Capsule 250 mg (metyrosine), Chemotherapy such as ENDOXAN, ONCOVIN, and Dacarbazine Injection and emerging drug includes ONC201, WELIREG, LUTATHERA, and others. The drug chapter also helps understand the Pheochromocytomas and Paragangliomas clinical trial details, expressive pharmacological action, agreements and collaborations, approval and patent details, and the latest news and press releases.
Pheochromocytomas and Paragangliomas Drugs
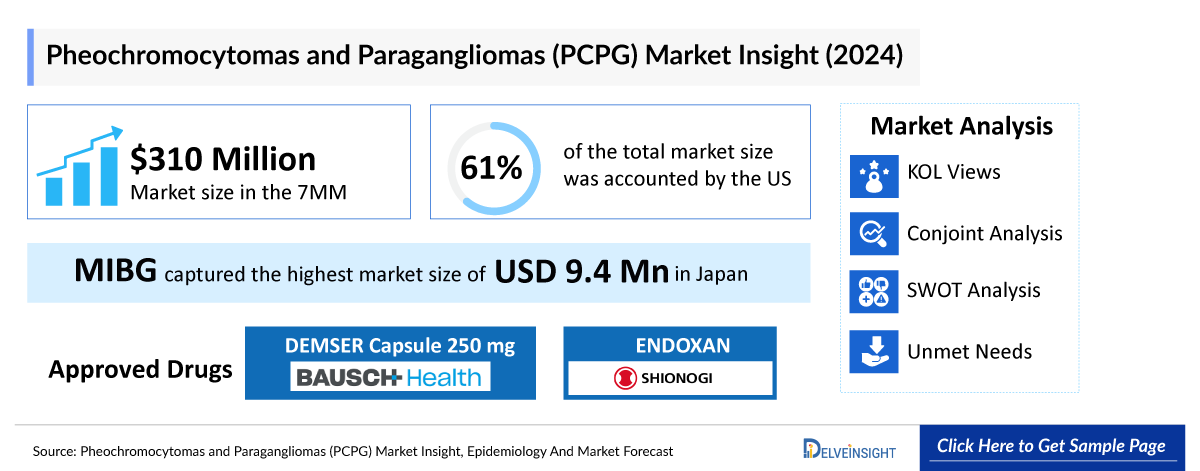
DEMSER Capsule 250 mg (metyrosine): Bausch Health and Ono Pharmaceutical
DEMSER is a promising drug with efficacy in the improvement of the symptoms in patients who are not able to sufficiently control the symptoms with sympatholytic drugs. DEMSER is indicated in the treatment of pheochromocytoma patients for preoperative preparation of patients for surgery, management of patients when surgery is contraindicated, and chronic treatment of malignant pheochromocytoma patients. DEMSER (ONO-5371) was approved and launched in the United States in 1979 for the treatment of pheochromocytoma. In January 2019, Ono Pharmaceutical received manufacturing and marketing approval in Japan for DEMSER capsule for improvement of the status of catecholamine excess secretion in pheochromocytoma patients. In July 2020, the US FDA approved Amneal Pharmaceuticals’ generic version of DEMSER (metyrosine) oral capsules. Amneal’s generic is the first for DEMSER to be approved in the United States.
|
Company |
RoA |
MoA |
|
Bausch Health and Ono Pharmaceutical |
Oral |
Tyrosine hydroxylase inhibitor |
|
Shionogi |
Intravenous Infusion |
Cross-linking of tumor cell DNA |
|
Nippon Kayaku |
Intravenous Infusion |
Inhibition of microtubule formation in mitotic spindle, resulting in an arrest of dividing cells at the metaphase stage |
|
Kyowa Hakko Kirin |
Intravenous Infusion |
DNA synthesis inhibition by its action as a purine analog, and interaction with SH groups |
Pheochromocytomas and Paragangliomas Emerging Drugs
ONC201: Chimerix
ONC201, also called dordaviprone, is a first-in-class small-molecule imipridone that selectively binds to the G-protein coupled dopamine receptor D2 (DRD2) and the mitochondrial protease ClpP. ONC201 has been evaluated in an open-label Phase II investigator-initiated study that treated 30 patients at the Cleveland Clinic with rare neuroendocrine tumors. Paraganglioma patients were enrolled in two cohorts initiating ONC201 either once or twice weekly. ONC201 interim efficacy results in dopamine-secreting tumors outside the brain showed 50% ORR in paraganglioma. Investigator-assessed data from this study were presented at the annual meeting of the American Society of Clinical Oncology (ASCO) in 2021 and published in the journal Clinical Cancer Research in 2022.
In January 2021, Chimerix acquired Oncoceutics, a privately held, clinical-stage biotechnology company developing imipridones to expand the pipeline with a late-stage oncology program.
WELIREG (belzutifan/MK-6482): Merck
WELIREG is a novel, potent and selective inhibitor of HIF-2a. WELIREG is indicated for von Hippel-Lindau (VHL) disease who need treatment for a type of kidney cancer called Renal Cell Carcinoma (RCC), tumors in the brain and spinal cord called Central Nervous System (CNS) hemangioblastomas, or a type of pancreatic cancer called pancreatic Neuroendocrine Tumors (pNET), that do not require surgery right away, and kidney cancer that has spread (advanced RCC) following treatment with a PD-1 or PD-L1 and VEGF cancer medicines.
It is currently being evaluated in Phase II for the treatment of advanced PCPG, with a target action date of May 26, 2025, under the Prescription Drug User Fee Act (PDUFA).
|
Company |
Mechanism of Action |
Phase |
Indication |
|
Chimerix |
A highly selective antagonist of dopamine receptor D2 (DRD2) and ClpP agonist |
II |
Metastatic neuroendocrine tumors, including PCPG and other rare NET types |
|
Merck |
HIF-2α inhibitor |
II |
Advanced PCPG |
|
Novartis |
Target somatostatin receptor (SSTR) |
II |
Somatostatin receptor-positive GEP-NETs and PCPG |
|
Perspective Therapeutics |
SSTR Subtype 2 (SSTR2) targeting peptide |
I/II |
Unresectable or metastatic somatostatin receptor type 2 -expressing NETs-PCPG |
Pheochromocytoma and Paraganglioma Drug Class Analysis
Pheochromocytoma and Paraganglioma are treated with various drug classes depending on the tumor's genetic profile and disease stage. Standard options include chemotherapy regimens such as CVD. Currently, clinical trials are exploring various targeted therapies to enhance treatment outcomes. These investigations include radionuclide therapies with SSTR2 agonists/antagonists and alpha-emitters, cold SSTR2 analogs (such as LUTATHERA and VMT-??-NET), HIF-2a inhibitors (WELIREG) and Dopamine Receptor D2 (DRD2) and ClpP agonist (ONC201). Each of these therapies offers unique mechanisms and potential advantages, positioning them as significant contenders in the evolving landscape of Pheochromocytoma and Paraganglioma treatment.
Note: Detailed insights will be provided in the final report...
Pheochromocytomas and Paragangliomas Market Outlook
For localized PCPG, surgery remains the mainstay of treatment. In cases of advanced or metastatic disease, treatment options include symptomatic therapy (alpha-blockers, beta-blockers, and catecholamine synthesis inhibitors) for functional PCPG in order to prevent life-threatening events. Additionally, systemic treatments like chemotherapy, targeted therapies, Somatostatin Analogs (SSAs), radiometabolic therapy, and loco-regional procedures such as cytoreductive surgery, external beam radiotherapy, arterial embolization, cryotherapy, and radiofrequency ablation are considered.
DEMSER (metyrosine) was approved in the United States in 1979 for treating pheochromocytoma. This FDA-approved medication is used for preoperative preparation, managing patients who cannot undergo surgery, and for the chronic treatment of malignant pheochromocytoma. Metastatic Pheochromocytomas and Paragangliomas management remains challenging, as there are only a few practiced standards of systemic therapy that were extensively adopted outside of controlled clinical trials. In July 2018, the FDA approved AZEDRA for treating PCPG, based on Study IB12B, a trial for patients with iobenguane scan-positive, unresectable, locally advanced, or metastatic PCPG. However, on August 15, 2023, Lantheus announced the discontinuation of AZEDRA production and the winding down of its Somerset, New Jersey manufacturing site. The decision to discontinue AZEDRA, the only approved therapy for PCPG, was driven by insufficient commercial demand, with manufacturing continuing only until Q1 2024.
Currently, clinical trials are exploring various targeted therapies to enhance treatment outcomes. These investigations include radionuclide therapies with SSTR2 agonists/antagonists and alpha-emitters, cold SSTR2 analogs (such as LUTATHERA and VMT-??-NET), HIF-2a inhibitors (like WELIREG (belzutifan)), and Dopamine Receptor D2 (DRD2) and ClpP agonist (ONC201). Each of these therapies offers unique mechanisms and potential advantages, positioning them as significant contenders in the evolving landscape of PCPG treatment.
- In 2024, MIBG captured the highest market size of approximately USD 9.4 million in Japan.
- In May 2025, Merck (NYSE: MRK) announced FDA approval of WELIREG® (belzutifan), an oral HIF-2α inhibitor, for treating adults and children 12+ with locally advanced, unresectable, or metastatic pheochromocytoma or paraganglioma (PPGL), rare tumors linked to genetic mutations. Approval was based on the LITESPARK-015 trial demonstrating objective response rate.
- In January 2025, the US FDA accepted for Priority Review a sNDA seeking approval of WELIREG, for the treatment of adult and pediatric patients (12 years and older) with advanced, unresectable, or metastatic PCPG. The FDA has set a PDUFA, or target action, date of May 26, 2025.
- In November 2024, Perspective Therapeutics presented the initial results from its ongoing Phase I/IIa clinical trial of [212Pb] VMT-a-NET at the 2024 NANETS Multidisciplinary NET Medical Symposium, held from November 21 to 23 in Chicago.
Pheochromocytomas and Paragangliomas Drugs Analysis
This section focuses on the uptake rate of potential drugs expected to be launched in the market during 2024–2034. The landscape of Pheochromocytomas and Paragangliomas treatment has experienced a profound transformation with the uptake of novel drugs. These innovative therapies are redefining standards of care. Furthermore, the increased uptake of these transformative drugs is a testament to the unwavering dedication of physicians, oncology professionals, and the entire healthcare community in their tireless pursuit of advancing cancer care. This momentous shift in treatment paradigms is a testament to the power of research, collaboration, and human resilience. Among the emerging therapies, WELIREG is expected to capture the largest pheochromocytomas and paragangliomas market size by 2034 in the United States.
Further detailed analysis of emerging therapies' drug uptake in the report…
Pheochromocytomas and Paragangliomas Pipeline Development Activities
The report provides insights into Pheochromocytomas and Paragangliomas clinical trials within Phase II, Phase I/II, and Phase I. It also analyzes key players involved in developing targeted therapeutics.
Pipeline Development Activities
The report covers information on collaborations, acquisitions and mergers, licensing, and patent details for pheochromocytomas and paragangliomas emerging therapy.
Latest KOL Views
To keep up with current market trends, we take KOLs and SMEs’ opinions working in the domain through primary research to fill the data gaps and validate our secondary research. Industry Experts were contacted for insights on the PCPG evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake, along with challenges related to accessibility, including oncologists, radiation oncologists, surgical oncologists, and others.
Delveinsight’s analysts connected with 30+ KOLs to gather insights; however, interviews were conducted with 15+ KOLs in the 7MM. Centers such as Technische Universität Dresden, Cancer Research Institute at Beth Israel Deaconess Medical Center, Aix-Marseille University, University of Oxford, Endocrinology and Diabetes Center, Cambridge University, University of Tsukuba etc., were contacted. Their opinion helps understand and validate current and emerging therapy treatment patterns or PCPG market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
What KOLs are saying on Pheochromocytomas and Paragangliomas Patient Trends?
- “Cluster-specific management regarding patient education, diagnostics (biochemistry, imaging), and follow-up are already widely acknowledged. Nevertheless, cluster-specific, genetically driven therapy requiring next-generation sequencing of individual tumors (with possibly single-cell sequencing) will very likely be an essential part of the management of these tumors in the future.”
- “In patients with a unilateral pheochromocytoma and no personal or family history suggestive of hereditary disease, genetic testing can be considered if patients are between the ages of 40 years and 50 years, but genetic testing is generally not recommended if patients are older than 50 years.”
Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the Analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving treatment landscape.
Market Access and Reimbursement
Market access and reimbursement for Pheochromocytoma and Paraganglioma (PCPG) present significant challenges due to the rarity and complexity of these conditions. Coverage for treatments and diagnostic procedures can be limited, impacting patient access to care. High costs associated with advanced therapies, such as peptide receptor radionuclide therapy (PRRT) and targeted radiopharmaceuticals, often lead to significant out-of-pocket expenses for patients. Insurance companies may be reluctant to cover treatments that are still under investigation or are not widely used, leading to disparities in access.
Living with a family member who has pheochromocytoma and paragangliomas often brings extra financial burdens. Any additional financial support the patient receive would be greatly appreciated and would help ease these challenges. To confirm the occurrence of the disease, the genetic counselor will determine the correct panel to order based on the patient personal family history. The test can cost between USD 250–1,000 and is usually covered by insurance, depending on the health plan of the patient.
Genetic Test
|
CPT code* |
|
81403 |
|
81404 |
|
81404 |
|
81404 |
|
81404 |
|
81405 |
|
81405 |
|
81405 |
|
81406 |
|
81406 |
|
81408 |
Scope of the Pheochromocytomas and Paragangliomas Market Report
- The report covers a segment of key events, an executive summary, and a descriptive overview of PCPG, explaining its causes, signs, symptoms, pathogenesis, and currently used therapies.
- Comprehensive insight into the epidemiology segments and forecasts, disease progression, and treatment guidelines has been provided.
- Additionally, an all-inclusive account of the emerging therapies and the elaborative profiles of mid-stage and prominent therapies will impact the current treatment landscape.
- A detailed review of the PCPG market, historical and forecasted market size, market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The report provides an edge while developing business strategies, by understanding trends, through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help shape and drive the 7MM PCPG market.
Pheochromocytomas and Paragangliomas Market Report Insights
- Pheochromocytomas and Paragangliomas Patient Population
- Pheochromocytomas and Paragangliomas Therapeutic Approaches
- Pheochromocytomas and Paragangliomas Pipeline Analysis
- Pheochromocytomas and Paragangliomas Market Size and Trends
- Existing and Future Pheochromocytomas and Paragangliomas Market Opportunities
Pheochromocytomas and Paragangliomas Market Report Key Strengths
- Ten-Year Forecast
- The 7MM Coverage
- Pheochromocytomas and Paragangliomas Epidemiology Segmentation
- Key Cross Competition
- Pheochromocytomas and Paragangliomas Drugs Uptake
- Key Pheochromocytomas and Paragangliomas Market Forecast Assumptions
Pheochromocytomas and Paragangliomas Market Report Assessment
- Current Pheochromocytomas and Paragangliomas Treatment Practices
- Pheochromocytomas and Paragangliomas Unmet Needs
- Pheochromocytomas and Paragangliomas Pipeline Product Profiles
- Pheochromocytomas and Paragangliomas Market Attractiveness
- Qualitative Analysis (SWOT Analysis and Conjoint Analysis)
- Pheochromocytomas and Paragangliomas Market Drivers
- Pheochromocytomas and Paragangliomas Market Barriers
FAQs answered through out Pheochromocytomas and Paragangliomas Market Report:
- What was the PCPG market size, the market size by therapies, market share (%) distribution in 2024, and what would it look like by 2034? What are the contributing factors for this growth?
- What are the pricing variations among different geographies for approved therapies?
- What can be the future treatment paradigm of PCPG?
- What are the disease risk, burdens, and unmet needs of PCPG? What will be the growth opportunities across the 7MM concerning the patient population with PCPG?
- What are the current options for the treatment of PCPG? What are the current guidelines for treating PCPG in the US, Europe, and Japan?
- What are the recent novel therapies, targets, mechanisms of action, and technologies being developed to overcome the limitation of existing therapies?
Reasons to Buy the Pheochromocytomas and Paragangliomas Market Report
- The report will help develop business strategies by understanding the latest trends and changing treatment dynamics driving the PCPG market.
- Insights on patient burden/disease prevalence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years.
- Understand the existing market opportunity in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
- Identifying strong upcoming players in the market will help devise strategies to help get ahead of competitors.
- Detailed analysis and ranking of class-wise potential current and emerging therapies under the Analyst view section to provide visibility around leading classes.
- Highlights of access and reimbursement policies of current therapies, barriers to accessibility of expensive off-label therapies, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing market so that the upcoming players can strengthen their development and launch strategy.
%20Market.png&w=256&q=75)