Psoriatic Arthritis Market
- The current Psoriatic Arthritis treatment market landscape includes medications such as HUMIRA (adalimumab), OTEZLA (apremilast), COSENTYX (secukinumab), CIMZIA (certolizumab pegol), and BIMZELX (bimekizumab), among others.
- Several promising Psoriatic Arthritis therapies, including SOTYKTU (deucravacitinib), ILUMYA (tildrakizumab), and others, are currently in late-stage clinical development.
- For PsA patients who do not respond to TNFi monotherapy, IL-17 inhibitors are typically favored over IL-12/23 inhibitors, abatacept, or tofacitinib. However, IL-12/23 inhibitors serve as an alternative for patients with coexisting IBD or those seeking less frequent dosing than IL-17 inhibitors.
- The leading Psoriatic Arthritis companies working in the Psoriatic Arthritis Market include bimekizumab (UCB Biopharma), tildrakizumab (Sun Pharmaceutical), deucravacitinib (BMS), and izokibep (Affibody AB)., and others.
Request a sample to unlock the CAGR for the Psoriatic Arthritis Drugs Market
DelveInsight’s ‘Psoriatic Arthritis Market Insights, Epidemiology, and Market Forecast – 2034’ report delivers an in-depth understanding of the historical and forecasted epidemiology as well as the psoriatic arthritis therapeutics market trends in the United States, EU5 (Germany, Spain, Italy, France, and the United Kingdom), and Japan.
The psoriatic arthritis market report provides current treatment practices, emerging Psoriatic Arthritis drugs, market share of the individual therapies, and current and forecasted psoriatic arthritis market size from 2020–2034 segmented by 7MM. The report also covers the current psoriatic arthritis treatment market practice/algorithm, Psoriatic Arthritis market drivers, market barriers, and Psoriatic Arthritis unmet needs to curate the best opportunities and assess the underlying market potential.
|
Study Period |
2020 to 2034 |
|
Forecast Period |
2024-2034 |
|
Geographies Covered |
|
|
Psoriatic Arthritis Market |
|
|
Psoriatic Arthritiss Market Size | |
|
Psoriatic Arthritis Companies |
UCB Biopharma, Sun Pharmaceutical Industries Limited, Bristol Myers Squibb, Affibody AB, Janssen Biotech, Amgen, Eli Lilly and Company, AbbVie, Pfizer, and others. |
|
Psoriatic Arthritis Epidemiology Segmentation |
|
Psoriatic Arthritis Treatment Market
Psoriatic Arthritis Overview
Psoriatic Arthritis is a form of arthritis associated with psoriasis, chronic skin and nail disease characterized by red, scaly rashes and thick pitted fingernails. Psoriatic Arthritis resembles rheumatoid arthritis (RA) in symptoms characterized by joint inflammation. However, Psoriatic Arthritis affects fewer joints than RA and does not produce the typical RA antibodies.
In 1956, Wright described arthritis associated with psoriasis. However, it was not until 1973 that Moll and Wright defined the various clinical phenotypes, including axial PsA, symmetrical polyarthritis, asymmetrical oligoarthritis, distal interphalangeal (DIP) arthritis, and arthritis mutilans. The following year, these authors introduced the concept of spondyloarthritis, a cluster of diseases with shared clinical and immunogenetic features. Despite these advances, the immunopathogenesis of Psoriatic Arthritis remained poorly understood, awaiting a more detailed understanding of immune networks and the inflammatory response.
The etiology and pathogenesis of Psoriatic Arthritis involve a complex interaction between genetic and environmental factors resulting in immune-mediated inflammation involving the skin and joints and may involve other organs. Approximately 33–50% of Psoriatic Arthritis patients have at least one first-degree relative with psoriasis or Psoriatic Arthritis. Genes associated with Psoriatic Arthritis include those in the HLA region involved in antigen presentation and immune recognition and non-HLA genes involved in immune activation and inflammation, including intracellular signaling, cytokine expression, signaling, and T-cell effector function. The role of environmental factors is suspected but has been difficult to confirm. Skin trauma induces psoriatic skin lesions flares, known as the Koebner phenomenon. Evidence suggests that joint trauma may cause a flare of arthritis, referred to as the “internal” or “deep” Koebner phenomenon.
Psoriatic Arthritis shares some clinical features with other inflammatory arthritides, including RA, reactive arthritis (ReA), and Ankylosing Spondylitis (AS). In some cases, it is difficult to make a precise diagnosis. Unlike Psoriatic Arthritis, RA is symmetrical and generally spares the DIP joints. AS has an earlier onset age than Psoriatic Arthritis, and sacroiliac involvement is usually symmetric rather than asymmetric.
Psoriatic Arthritis may range from mild to severe, and treating it no matter the severity is crucial. If left untreated, Psoriatic Arthritis can cause permanent joint damage, which may be disabling. In addition to preventing irreversible joint damage, treating Psoriatic Arthritis may also help reduce inflammation that could lead to other comorbidities. However, no cure for Psoriatic Arthritis exists, so treatment goals are to slow disease progression, improve QoL, lessen pain, and preserve the range of motion. In most Psoriatic Arthritis patients, pharmacological treatment consists of a trial-and-error approach, beginning with corticosteroids and nonsteroidal anti-inflammatory drugs to manage symptoms. Physicians often use conventional synthetic disease-modifying antirheumatic drugs (DMARDs), followed by biological DMARDs, if a patient does not respond adequately.
Psoriatic Arthritis Diagnosis and Treatment
It covers the details of conventional and current medical therapies and diagnoses available in the Psoriatic Arthritis market to treat the condition. It also provides country-wise treatment guidelines and algorithms across the United States, Europe, and Japan.
Psoriatic Arthritis Epidemiology
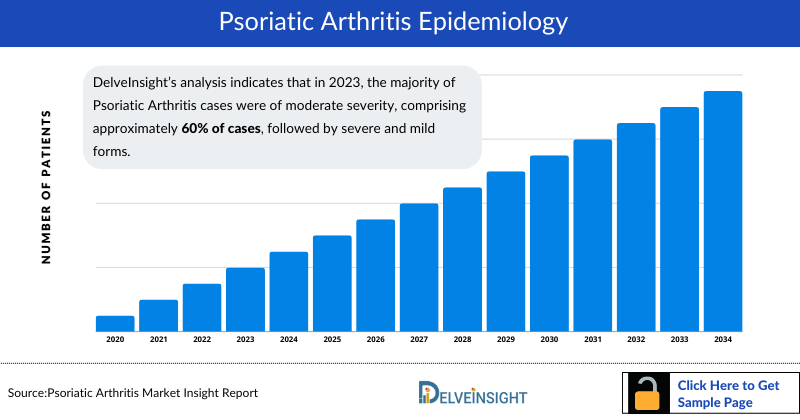
The Psoriatic arthritis epidemiology division provides insights into the historical and current psoriatic arthritis patient pool and the forecasted trend for every 7MM. It helps recognize the causes of current and forecasted trends by exploring numerous studies and views of KOL. The report also provides the prevalent patient pool, trends, and assumptions.
Psoriatic Arthritis Key Findings
The disease epidemiology covered in the report provides historical and forecasted Psoriatic Arthritis epidemiology segmented as the prevalent cases of Psoriatic Arthritis, diagnosed cases of Psoriatic Arthritis, gender-specific cases of Psoriatic Arthritis, age-specific cases of Psoriatic Arthritis and severity-specific cases of Psoriatic Arthritis. The report includes the prevalent Psoriatic Arthritis scenario in the 7MM covering the United States, EU5 countries (Germany, France, Italy, Spain, and the United Kingdom), and Japan from 2020 to 2034.
Country-wise Psoriatic Arthritis Epidemiology
The epidemiology segment also provides psoriatic arthritis epidemiology data and findings across the United States, EU5 (Germany, France, Italy, Spain, and the United Kingdom), and Japan.
Key Findings:
- According to DelveInsight's analysis, Psoriatic Arthritis was observed to affect females more frequently than males. In 2023, females accounted for approximately 15% more PsA cases compared to males.
- DelveInsight’s analysis indicates that in 2023, the majority of Psoriatic Arthritis cases were of moderate severity, comprising approximately 60% of cases, followed by severe and mild forms.
- In the US in 2023, the age group 50–59 years had the highest prevalence of Psoriatic Arthritis, while the 18–29 age group had the lowest number of cases.
- According to DelveInsight's analysis, in 2023, Germany had the highest number of Psoriatic Arthritis cases among the EU4 countries and the UK, while Spain reported the lowest.
- The National Psoriatic Foundation reports that approximately 1 in 3 individuals, or 30%, with psoriasis develop Psoriatic Arthritis, affecting up to 2.4 million Americans. PsA can occur at any age.
- According to the study by Brent et al. (2024), the prevalence of psoriatic arthritis among individuals with psoriasis was 1.7% at 5 years, 3.1% at 10 years, 5.1% at 20 years, and 20.5% at 30 years. Overall, the prevalence of psoriatic arthritis in psoriasis patients is 19.7%, with 21.6% in adults and 3.3% in children.
Unlock comprehensive insights! Click Here to Purchase the Full Epidemiology Report @ Psoriatic Arthritis Prevalence
Psoriatic Arthritis Recent Developments
- In May 2025, Teva Pharmaceuticals and Alvotech announced that the FDA has approved SELARSDI™ (ustekinumab-aekn) injection as interchangeable with the reference biologic Stelara® (ustekinumab). Starting April 30, 2025, SELARSDI is available in all presentations matching the reference product, for the treatment of adults and pediatric patients with psoriatic arthritis, plaque psoriasis, Crohn’s disease, and ulcerative colitis.
- In March 2025, Celltrion announced the U.S. launch of STEQEYMA® (ustekinumab-stba), a biosimilar to STELARA® (ustekinumab), following FDA approval in December 2024. STEQEYMA is approved for the same indications as STELARA, offering consistent treatment for patients and healthcare providers.
- In December 2024, Celltrion announced that the FDA approved STEQEYMA® (ustekinumab-stba), a biosimilar to STELARA® (ustekinumab), for subcutaneous injection or intravenous infusion. It is approved for adult and pediatric patients with plaque psoriasis and psoriatic arthritis, as well as adult patients with Crohn's disease and ulcerative colitis.
- In October 14, 2024, Dong-A-ST announced that its Imuldosa (ustekinumab-srlf/DMB-3115), a biosimilar to Stelara, received FDA approval for the treatment of autoimmune diseases such as plaque psoriasis, psoriatic arthritis, Crohn’s disease, and ulcerative colitis. Stelara, a blockbuster drug developed by Janssen Biotech, generated $10.86 billion in sales globally in 2023, and Imuldosa’s approval as a biosimilar offers a more cost-effective alternative for patients requiring long-term therapy for autoimmune conditions.
- In September 23rd, 2024, UCB announced that the FDA had approved BIMZELX (bimekizumab-bkzx) for adults with active psoriatic arthritis, active non-radiographic axial spondyloarthritis with objective signs of inflammation, and active ankylosing spondylitis. This makes BIMZELX the first and only IL-17A and IL-17F inhibitor approved in the U.S. for these four chronic immune-mediated inflammatory diseases.
- In February 2024, The FDA has accepted UCB's supplemental Biologics License Application (sBLA) for bimekizumab, targeting three new indications, including Psoriatic Arthritis. This development follows the outcomes of four Phase III clinical trials evaluating bimekizumab's effectiveness in PsA, ankylosing spondylitis, and non-radiographic axial spondyloarthritis
Psoriatic Arthritis Drug Chapters
The drug chapter segment of the psoriatic arthritis market research report encloses a detailed analysis of marketed Psoriatic Arthritis drugs and late-stage (Phase III, Phase II/III, Phase II, and Phase I/II) psoriatic arthritis pipeline drugs. It also helps understand Psoriatic Arthritis clinical trials details, expressive pharmacological action, agreements and collaborations, approval and patent details, advantages and disadvantages of each included drug, and the latest psoriatic arthritis news and press releases.
The current standard of care lacks efficiency in treating Psoriatic Arthritis that permanently cures PsA and demands need to develop more effective therapies for PsA. Although many effective PsA therapies, including csDMARDS, TNF inhibitors, various anti-IL inhibitors, and a variety of small molecule therapeutics, have been introduced over the last decade, spanning several different mechanisms of action. Still, many patients often do not respond to treatment due to various resistance symptoms. In addition to this, concerns over long-term safety profiles of csDMARDS and currently available biologics may limit their use, and patients can find treatments difficult due to regular blood monitoring, concerns of side effects, method of administration as well as the impact on metabolic risk, which in turn can lead to poor compliance and dissatisfaction.
Products detail in the report…
Emerging Psoriatic Arthritis Drugs
Drug developers are gradually shifting their attention toward Psoriatic Arthritis to meet the patient pool’s current demands and counter the unmet needs of the therapeutic market.
Several Psoriatic Arthritis companies are working robustly on many new therapies, such as bimekizumab (UCB Biopharma), tildrakizumab (Sun Pharmaceutical), deucravacitinib (BMS), and izokibep (Affibody AB).
Bimekizumab (UCB Biopharma) is an investigational, humanized monoclonal IgG1 antibody that selectively inhibits both IL-17A and IL-17F, two key cytokines driving inflammatory processes. IL-17F has overlapping biology with IL-17A and drives inflammation independently of IL-17A.
The safety and efficacy of bimekizumab are being evaluated across multiple disease states as part of a robust clinical program. The company is evaluating the drug in multiple Phase III Psoriatic Arthritis clinical studies for PsA.
Tildrakizumab (Sun Pharmaceutical) is a humanized lgG1/k monoclonal antibody designed to selectively bind to the p19 subunit of IL-23 (IL-23) and inhibit its interaction with the IL-23 receptor, leading to inhibition of the release of pro-inflammatory cytokines and chemokines. ILUMYA is indicated for the treatment of adults with moderate-to-severe plaque psoriasis who are candidates for systemic therapy or phototherapy in the US.
The drug is currently being evaluated in Phase III Psoriatic Arthritis clinical trials in subjects with active Psoriatic Arthritis with prior exposure to an anti-TNF agent(s) as well as in anti-TNF naïve subjects with active Psoriatic Arthritis.
The company has filled marketing application for the manufacturing and marketing authorization of tildrakizumab for moderate-to-severe psoriasis and Psoriatic Arthritis in Japan.
Products detail in the report…
Psoriatic Arthritis Market Outlook
The Psoriatic arthritis market outlook builds a detailed comprehension of the historical, current, and forecasted psoriatic arthritis therapeutics market trends by analyzing the impact of current therapies on the market, unmet needs, and demand for better technology.
This segment gives a thorough detail of the psoriatic arthritis therapeutics market trend of each marketed drug and late-stage pipeline therapy by evaluating their impact based on the annual cost of therapy, inclusion and exclusion criteria, mechanism of action, compliance rate, growing need for the market, increasing patient pool, covered patient segment, expected launch year, competition with other therapies, brand value, their impact on the Psoriatic Arthritis treatment market, and KOL view. The calculated psoriatic arthritis market outlook data are presented with relevant tables and graphs to give a clear view of the market at first sight.
As per DelveInsight, the psoriatic arthritis market size in 7MM is expected to change in the study period 2020–2034.
Key Findings
This section includes a glimpse of the psoriatic arthritis treatment market in 7MM. In the 7MM, psoriatic arthritis market size was approximately USD 9 billion in 2021.
The United States: Psoriatic Arthritis Market Outlook
This section provides the total psoriatic arthritis market size. It also provides the market size of psoriatic arthritis by systemic therapies and by-products in the United States.
The United States accounts for the highest psoriatic arthritis market size than the EU5 (the United Kingdom, Germany, Italy, France, and Spain) and Japan.
EU-5 Countries: Psoriatic Arthritis Market Outlook
This section provides the total psoriatic arthritis market size. It also provides the market size of Psoriatic Arthritis by systemic therapies and by-products in Germany, France, Italy, Spain, and the United Kingdom.
Japan: Psoriatic Arthritis Market Outlook
This section provides the total psoriatic arthritis market size. It also provides the market size of Psoriatic Arthritis by systemic therapies and by-products in Japan.
Psoriatic Arthritis Drugs Uptake
This section focuses on the uptake rate of potential Psoriatic Arthritis drugs recently launched or expected to be launched in the Psoriatic Arthritis treatment market during the study period 2020–2034. The analysis covers Psoriatic Arthritis therapeutics market uptake by drugs, patient uptake by therapies, and sales of each drug.
This helps in understanding the Psoriatic Arthritis drugs with the most rapid uptake and the reasons behind the maximal use of new psoriatic arthritis drugs and allows the comparison of the Psoriatic Arthritis drugs based on market share and size, which again will be useful in investigating factors important in the market uptake and in making financial and regulatory decisions.
Psoriatic Arthritis Pipeline Development Activities
The Psoriatic Arthritis pipeline segment provides insights into Psoriatic Arthritis clinical trials within Phase III, Phase II/III, Phase II, and Phase I/II stages. It also analyses Psoriatic Arthritis companies in developing targeted therapeutics.
Major Psoriatic Arthritis companies include Sun Pharmaceuticals, Bristol Myers Squibb, Affibody AB, UCB Biopharma and others whose key products are expected to get launched in the US market by 20XX.
Pipeline Development Activities
The Psoriatic Arthritis pipeline segment covers information on collaborations, acquisitions, mergers, licensing, and patent details for emerging Psoriatic Arthritis therapies.
Take Your Research to the Next Level! Click Here to Get Access to the Full Pipeline Report @ Psoriatic Arthritis Drugs
KOL Views
To keep up with current market trends, we take KOLs and SMEs’ opinions working in Psoriatic Arthritis domain through primary research to fill the data gaps and validate our secondary research. Their opinion helps understand and validate current and emerging therapies treatment patterns or Psoriatic Arthritis market trends. This will support the clients in potential novel treatments by identifying the overall scenario of the market and the Psoriatic Arthritis unmet needs.
Competitive Intelligence Analysis
We perform a competitive and market intelligence analysis of Psoriatic Arthritis market using various competitive intelligence tools: SWOT analysis, PESTLE analysis, Porter’s five forces, BCG Matrix, Market entry strategies, etc. The inclusion of the analysis entirely depends upon the data availability.
Psoriatic Arthritis Market Research Report Scope
- The report covers the descriptive overview of Psoriatic Arthritis, explaining its causes, signs and symptoms, pathophysiology, and currently available Psoriatic Arthritis therapies
- Comprehensive insight is provided into Psoriatic Arthritis epidemiology and treatment in the 7MM
- Additionally, an all-inclusive account of both the current and emerging Psoriatic Arthritis therapies for Psoriatic Arthritis is provided, along with the assessment of new Psoriatic Arthritis therapies that will impact the current treatment landscape
- A detailed review of the Psoriatic Arthritis market; historical and forecasted, is included in the report, covering drug outreach in the 7MM
- The patient-based Psoriatic Arthritis market forecasting report provides an edge while developing business strategies by understanding trends shaping and driving the global Psoriatic Arthritis market
Psoriatic Arthritis Market Report Highlights
- Recently, the Psoriatic Arthritis market is set to change due to the rising awareness of the disease and incremental healthcare spending across the world; which would expand the size of the market to enable the drug manufacturers to penetrate more into the market
- The Psoriatic Arthritis companies and academics are working to assess challenges and seek opportunities that could influence Psoriatic Arthritis R&D. The Psoriatic Arthritis emerging Psoriatic Arthritis therapies under development are focused on novel approaches to treat/improve the disease condition
- Major players are involved in developing Psoriatic Arthritis therapies for Psoriatic Arthritis. The launch of emerging Psoriatic Arthritis therapies will significantly impact the Psoriatic Arthritis market
- For Psoriatic Arthritis, a better understanding of disease pathogenesis will also contribute to developing novel therapeutics
- Our in-depth analysis of the pipeline assets across different stages of development (Phase III and Phase II), different emerging trends, and comparative analysis of pipeline products with detailed Psoriatic Arthritis clinical trials, key cross-competitor, launch date along with product development activities will support the clients in the decision-making process regarding their therapeutic portfolio by identifying the overall scenario of the R&D activities
Psoriatic Arthritis Market Report Insights
- Patient-based Psoriatic Arthritis Market Forecasting
- Psoriatic Arthritis Therapeutic Approaches
- Psoriatic Arthritis Pipeline Drugs Analysis
- Psoriatic Arthritis Market Size
- Psoriatic Arthritis Market Trends
- Psoriatic Arthritis Market Opportunities
- Impact of Upcoming Psoriatic Arthritis Therapies
Psoriatic Arthritis Market Report Key Strengths
- 10-year- Psoriatic Arthritis Market Forecast
- 7MM Coverage
- Psoriatic Arthritis Epidemiology Segmentation
- Key Competitors
- Highly Analyzed Market
- Psoriatic Arthritis Drugs Uptake
Psoriatic Arthritis Treatment Market Report Assessment
- Current Psoriatic Arthritis Treatment Market Practices
- Psoriatic Arthritis Unmet Needs
- Psoriatic Arthritis Pipeline Product Profiles
- Psoriatic Arthritis Market Attractiveness
- Psoriatic Arthritis Market Drivers
- Psoriatic Arthritis Market Barriers
Key Questions Answered In The Psoriatic Arthritis Market Report:
Psoriatic Arthritis Treatment Market Insights:
- What was the market share percentage distribution, and how would it look by 2034?
- What would be the total Psoriatic Arthritis market size and market size of Psoriatic Arthritis therapies across the 7MM forecast period (2024–2034)?
- What are the key findings about the market across 7MM, and which country will have the largest Psoriatic Arthritis market size during the forecast period (2024–2034)?
- At what CAGR is the Psoriatic Arthritis market expected to grow in the 7MM forecast period (2024–2034)?
- What would be the Psoriatic Arthritis market outlook across the 7MM forecast period (2024–2034)?
- What would be the Psoriatic Arthritis market growth until 2034 and the resultant market size by 2034?
- How would future opportunities affect the market dynamics and subsequent analysis of the associated trends?
Psoriatic Arthritis Epidemiology Insights:
- What are the disease risks, burdens, and Psoriatic Arthritis unmet needs?
- What is the historical patient pool of Psoriatic Arthritis covering the United States, EU5 (Germany, Spain, France, Italy, UK), and Japan?
- What would be the forecasted patient pool of Psoriatic Arthritis covering the United States, EU5 (Germany, Spain, France, Italy, UK), and Japan?
- What will be the growth opportunities in the 7MM concerning the patient population of Psoriatic Arthritis?
- Out of all the 7MM, which country would have the highest prevalence of Psoriatic Arthritis during the forecast period (2024–2034)?
- At what CAGR is the population expected to grow in the 7MM during the forecast period (2024–2034)?
Current Psoriatic Arthritis Treatment Market Scenario, Marketed Drugs, and Emerging Therapies:
- What are the current options to treat Psoriatic Arthritis?
- What are the current treatment guidelines for treating Psoriatic Arthritis in the US, Europe, and Japan?
- What are marketed Psoriatic Arthritis drugs and their MOA, regulatory milestones, product development activities, advantages, disadvantages, safety, efficacy, etc.?
- How many companies are developing therapies to treat Psoriatic Arthritis?
- How many Psoriatic Arthritis therapies are developed by each company to treat Psoriatic Arthritis?
- How many emerging Psoriatic Arthritis therapies are in the mid-stage and late stages of development to treat Psoriatic Arthritis?
- What are the key collaborations (Industry–Industry, Industry-Academia), Mergers and acquisitions, and licensing activities related to Psoriatic Arthritis therapies?
- What are the recent novel Psoriatic Arthritis therapies, targets, mechanisms of action, and technologies developed to overcome the limitation of existing therapies?
- What are the Psoriatic Arthritis clinical trials and its status?
- Which key designations have been granted for the emerging Psoriatic Arthritis therapies?
- What are the global historical and forecasted Psoriatic Arthritis markets?
Reasons to Buy Psoriatic Arthritis Market Forecast Report
- The patient-based Psoriatic Arthritis market forecasting report will help in developing business strategies by understanding trends shaping and driving Psoriatic Arthritis market
- To understand the future market competition in the Psoriatic Arthritis market and an Insightful review of the key market drivers and barriers.
- Organize sales and marketing efforts by identifying the best opportunities for Psoriatic Arthritis in the US, Europe (Germany, Spain, Italy, France, and the United Kingdom), and Japan
- Identifying upcoming solid players in the market will help devise strategies that will help get ahead of competitors
- Organize sales and marketing efforts by identifying the best opportunities for the Psoriatic Arthritis market
- To understand the future market competition in the Psoriatic Arthritis market
Read Our Articles