BCL-2 Inhibitors Market Summary
- The B-Cell Leukemia/Lymphoma-2 Inhibitors market in the 7MM is projected to grow at a significant CAGR by 2034 in leading countries (US, EU4, UK and Japan).
- The BCL-2 inhibitors market is expected to grow significantly in the coming years. This is due to the increasing number of patients who are being diagnosed with cancer, the growing awareness of BCL-2 inhibitors, and the increasing number of BCL-2 inhibitors that are under clinical trials.
B-Cell Leukemia/Lymphoma-2 Inhibitors Market and Epidemiology Analysis
- BCL-2 is a protein that plays a critical role in the regulation of cell death, known as apoptosis. The overexpression of BCL-2 is frequently detected in numerous cancer types, which prevents apoptosis of cancer cells.
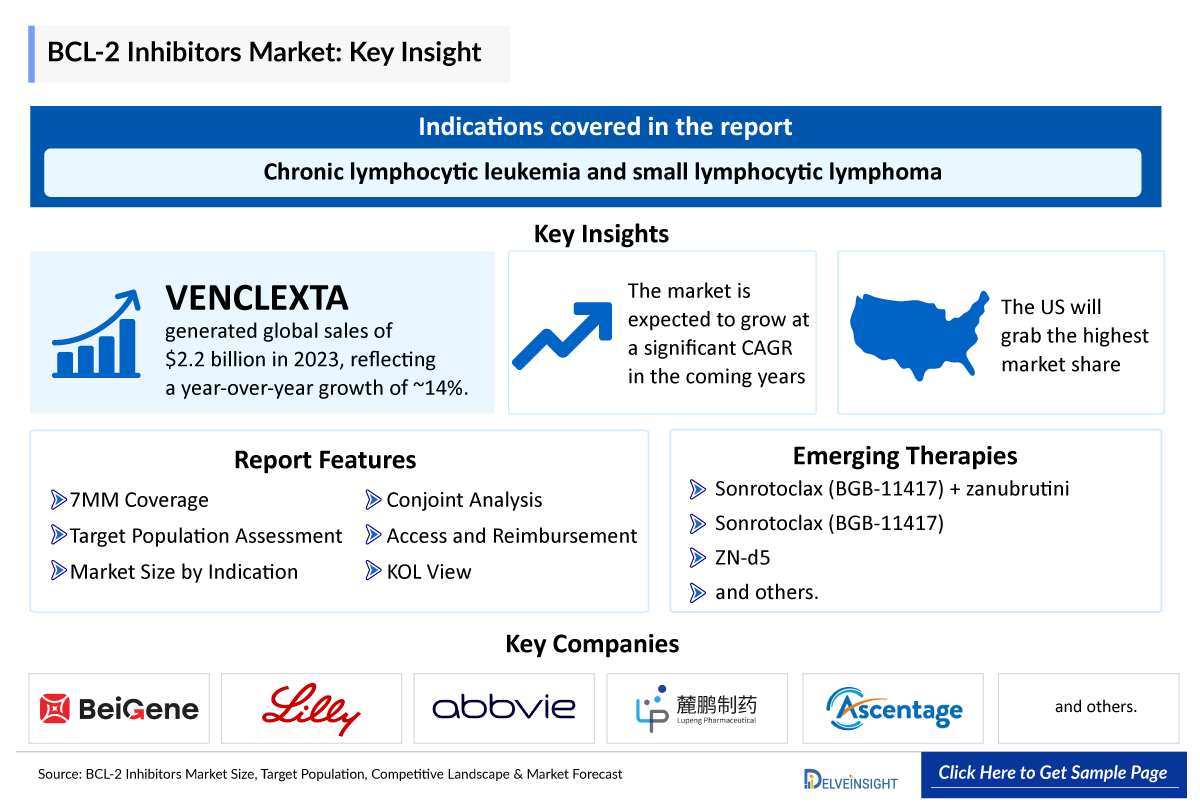
- In 2016, VENCLEXTA (venetoclax) became the first BCL-2 inhibitor to receive FDA approval as standard therapy for the treatment of chronic lymphocytic leukemia. Currently, it is also approved in combination with other agents to treat acute myeloid therapy and as monotherapy for treatment of chronic lymphocytic leukemia and small lymphocytic lymphoma.
- VENCLEXTA (venetoclax) is a first-in-class medicine that selectively binds and inhibits the B-cell lymphoma-2 (BCL-2) protein. In some blood cancers, BCL-2 prevents cancer cells from undergoing their natural death or self-destruction process, called apoptosis. VENCLYXTO targets the BCL-2 protein and works to help restore the process of apoptosis.
- In 2023, VENCLEXTA generated the global sales of USD 2.2 billion with growth rate of approximately 14% year on year basis.
- Several BCL-2 inhibitors are currently being evaluated in clinical trials. An example is BeiGene’s sonrotoclax (BGB-11417) which is in the developmental stage and is anticipated to receive approval during the forecast period.
- Sonrotoclax stands as the leading BCL-2 inhibitor in clinical advancement, presently undergoing in phase III trials as combination therapay with zanubrutini for the treatment of chronic lymphocytic leukemia and as a monotherapy for the treatment of various types of cancer in phase II.
- In October 2023, Ascentage Pharma and AstraZeneca entered into clinical collaboration for the study of BCL-2 inhibitor lisaftoclax (APG-2575) in combination with BTK Inhibitor acalabrutinib in treatment-naïve patients with first-line CLL/SLL.
- Many BCL-2 inhibitors companies such as Ascentage Pharma, who are working on BCL-2 inhibitor are expected to present the data of their BCL-2 inhibitors studies during Conferences such as the ASCO 2024 annual meeting and others.
- In May 2024, sonrotoclax developed by BeiGene received fast track designation for the treatment of mantle-cell lymphoma (monotherapy, second-line therapy or greater) in the US.
- In November 2023, FDA granted orphan drug designation to sonrotoclax for the treatment of multiple myeloma.
- In January 2024, innoCare announced clearance of investigational new drug application for clinical trial of BCL-2 Inhibitor ICP-248 by the FDA. ICP-248 is a novel, orally bioavailable BCL-2 selective inhibitor, investigated for the treatment of hematologic malignancies as a monotherapy or in combination with other therapies.
- BeiGene, Zentalis pharmaceuticals and several other BCL-2 inhibitors companies are currently engaged in the development and production of selective BCL-2 inhibitors, which have the potential to significantly impact and enhance the BCL-2 inhibitors market.
Request for unlocking the Sample Page of the BCL-2 Inhibitors Market
Factors Affecting BCL-2 Inhibitors Market Growth
Rising Prevalence of Hematological Malignancies
The increasing incidence of blood cancers such as chronic lymphocytic leukemia (CLL), acute myeloid leukemia (AML), and non-Hodgkin lymphoma is a primary driver of the BCL-2 inhibitors market, as these therapies play a critical role in apoptosis-based cancer treatment.
Strong Clinical Efficacy of Targeted Therapies
BCL-2 inhibitors offer targeted mechanisms of action with improved clinical outcomes compared to conventional chemotherapy. Their ability to induce programmed cell death in cancer cells has strengthened physician adoption and treatment guideline inclusion.
Expanding Clinical Pipeline and Ongoing Trials
A growing number of clinical trials evaluating BCL-2 inhibitors as monotherapies and in combination regimens is supporting market expansion. Pipeline diversification across indications and therapy lines is enhancing long-term growth potential.
Increased Use of Combination Therapies
The integration of BCL-2 inhibitors with BTK inhibitors, monoclonal antibodies, and chemotherapy regimens has improved treatment efficacy and broadened their use across multiple cancer settings, boosting market demand.
Favorable Regulatory Support
Regulatory incentives such as accelerated approvals, breakthrough therapy designations, and orphan drug status are facilitating faster market entry and encouraging continued innovation in the BCL-2 inhibitors space.
High Treatment Costs
The premium pricing of targeted oncology therapies remains a significant challenge. High costs may limit patient access in price-sensitive markets and create reimbursement hurdles, impacting overall market penetration.
Safety Concerns and Adverse Events
Potential side effects such as tumor lysis syndrome, cytopenias, and infection risks require careful monitoring, which may restrict use in certain patient populations and influence prescribing patterns.
Development of Drug Resistance
Emerging resistance mechanisms to BCL-2 inhibitors can reduce long-term treatment effectiveness, prompting the need for next-generation therapies and potentially slowing sustained market growth.
Competition from Alternative Targeted Therapies
The availability of other targeted agents and immunotherapies may limit the standalone use of BCL-2 inhibitors, increasing competitive pressure within the oncology therapeutics market.
Access and Infrastructure Limitations
Limited access to advanced oncology care, diagnostic tools, and specialized treatment centers in developing regions may restrict market growth despite rising disease prevalence.
DelveInsight’s “B-Cell Leukemia/Lymphoma-2 Inhibitors Market Size, Target Population, Competitive Landscape, and Market Forecast – 2034” report delivers an in-depth understanding of the BCL-2 inhibitors, historical and forecasted epidemiology, competitive landscape as well as the BCL-2 inhibitors market trends in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
The BCL-2 inhibitors Market Report provides current treatment practices, emerging drugs, market share of individual therapies, and current and forecasted 7MM BCL-2 inhibitors market size from 2020 to 2034. The BCL-2 inhibitors market report also covers current BCL-2 inhibitors treatment practices/algorithms and unmet medical needs to curate the best opportunities and assess the BCL-2 inhibitors market’s potential.
Scope of the BCL-2 inhibitors Market | |
|
Study Period |
2020 to 2034 |
|
Geographies Covered |
|
|
BCL-2 Inhibitors Companies |
|
BCL-2 Inhibitors Market: Understanding and Treatment Algorithm
BCL-2 is a constituent of the BCL-2 protein family, which plays a pivotal role in the modulation of apoptosis. The proteins within this particular family can be classified into two main groups: anti-apoptotic members, such as BCL-2 itself, BCL-XL, MCL1, and pro-apoptotic members, such as BAX, BAK, and BAD. BCL-2 inhibitors represent a class of drugs that target the B-cell lymphoma-2 (BCL-2) protein, which plays a pivotal role in regulating apoptosis, or programmed cell death. These inhibitors are primarily developed for the treatment of various cancers, particularly hematologic malignancies like chronic lymphocytic leukemia (CLL) and certain types of lymphomas.
BCL-2 Expression
Research has revealed that the over-expression of BCL-2 plays a significant role in the development of various cancer types, including B-cell lymphoma, prostate cancer, lung cancer, acute lymphoblastic leukemia, breast cancer, and others.
BCL-2 Inhibitors Treatment
BCL-2 inhibitors treatment, such as venetoclax, is primarily utilized in the management of certain hematologic malignancies, particularly those where dysregulation of apoptosis contributes to disease pathogenesis. BCL-2 inhibitors are approved for the treatment of specific hematologic malignancies, such as chronic lymphocytic leukemia, small lymphocytic lymphoma, and acute myeloid leukemia in specific patient populations. Patient selection for BCL-2 inhibitor therapy involves considerations such as disease type, stage, genetic markers, prior treatments, and overall health status. These inhibitors are often used in patients with relapsed or refractory disease, as well as in frontline therapy for certain indications.
BCL-2 inhibitors are frequently used in combination with other anti-cancer agents to enhance treatment efficacy. Combinations may include chemotherapy, immunotherapy, targeted therapies, or other novel agents. The choice of combination therapy depends on the specific cancer type and patient characteristics. During BCL-2 inhibitor treatment, patients undergo regular assessments to monitor treatment response and disease progression. This may involve imaging studies, blood tests, and clinical evaluations. Response criteria vary depending on the cancer type and treatment goals.
The duration of BCL-2 inhibitor treatment varies depending on factors such as disease response, tolerability, and treatment goals. Some patients may receive continuous therapy, while others may undergo intermittent treatment or transition to maintenance therapy after achieving remission. Overall, BCL-2 inhibitor treatment represents a targeted approach to inducing apoptosis in cancer cells, offering promising therapeutic options for patients with certain hematologic malignancies.
BCL-2 Inhibitors Drugs Analysis
The drug chapter segment of the BCL-2 inhibitors market reports encloses a detailed analysis of BCL-2 inhibitors marketed drugs and late-stage (Phase III and Phase II) pipeline drugs. It also helps understand the BCL-2 inhibitors' clinical trial details, expressive pharmacological action, agreements and collaborations, approval and patent details, advantages and disadvantages of each included drug and the latest news and press releases.
Marketed BCL-2 Inhibitor Drugs
-
VENCLEXTA (venetoclax): Abbvie
VENCLEXTA is the first FDA-approved treatment that targets the B-cell lymphoma 2 (BCL-2) protein, which supports cancer cell growth and is overexpressed in many patients with chronic lymphocytic leukemia. The FDA has granted VENCLEXTA breakthrough therapy designation, priority review status, and accelerated approval for CLL. Currently VENCLEXTA is approved by the FDA as a monotherapy for the treatment of chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) and as a Combination therapy with azacitidine, or decitabine, or low-dose cytarabine for the treatment of newly diagnosed acute myeloid leukemia (AML) VENCLEXTA is being developed by AbbVie and Roche. It is jointly commercialized by AbbVie and Genentech, a member of the Roche Group, in the US and by AbbVie outside of the US. Together, the companies are committed to BCL-2 research and to studying venetoclax in clinical trials across several blood and other cancers. Venetoclax is approved in more than 80 countries, including the US.
Analysis of Marketed BCL-2 Inhibitor Drugs | ||
|
Product |
Company |
Indication |
|
VENCLEXTA (venetoclax) |
Abbvie |
|
Emerging BCL-2 Inhibitor Drugs
-
Sonrotoclax: BeiGene
Sonrotoclax is an investigational small molecule B-cell lymphoma-2 inhibitor. It emerges as a potential second-generation BCL-2 inhibitor for the treatment of hematologic malignancies with the potential to overcome BCL-2 mutation-induced venetoclax resistance. It belongs to a class of BCL-2 homology 3 (BH3) mimetics, and preclinical and IND-enabling studies have demonstrated potent activity and high selectivity of sonrotoclax against the antiapoptotic protein BCL-2. Sonrotoclax is more potent and selective for BCL-2 relative to BCLxL than venetoclax. Sonrotoclax is currently under investigation in multiple clinical trials.
-
ZN-d5: Zentalis Pharmaceuticals
ZN-d5 is a selective, oral small molecule inhibitor of B-cell lymphoma-2 (BCL-2), which is currently being evaluated in patients with hematologic malignancies. It is designed by Zentalis pharmaceuticals to have best in class potency, selectivity and pharmacokinetic properties. Currently ZN-d5 is being studied in phase I/II study in combination with azenosertib in relapsed or refractory acute myeloid leukemia.
List of BCL-2 Inhibitor Emerging Drugs | |||||
|
Sonrotoclax (BGB-11417) + zanubrutini |
BeiGene |
Chronic lymphocytic leukemia |
BCL-2 inhibitor |
III |
NCT06073821 |
|
Sonrotoclax (BGB-11417) |
Relapsed or refractory chronic lymphocytic leukemia or small lymphocytic lymphoma |
BCL-2 inhibitor |
II |
NCT05479994 | |
|
Waldenstrom macroglobulinemia, waldenstrom's macroglobulinemia recurrent, waldenstrom's macroglobulinemia refractory |
BCL-2 inhibitor |
II |
NCT05952037 | ||
|
Mantle cell lymphoma, refractory mantle cell lymphoma (MCL), relapsed mantle cell lymphoma |
BCL-2 inhibitor |
I/II |
NCT05471843 | ||
|
ZN-d5 |
Zentalis pharmaceuticals |
Acute myeloid leukemia (AML) |
BCL-2 inhibitor |
I/II |
NCT05682170 |
BCL-2 Inhibitors Market Outlook
The BCL-2 inhibitors market is expected to grow significantly in the coming years. This is due to the increasing number of patients who are being diagnosed with cancer, the growing awareness of BCL-2 inhibitors, and the increasing number of BCL-2 inhibitors that are under clinical trials. Upon approval in 2016, venetoclax (VENCLEXTA) became the first BCL-2 inhibitor to receive FDA approval as standard therapy for the treatment of chronic lymphocytic leukemia who have a 17p deletion and have received at least one prior therapy. Over time, venetoclax's indications have expanded to include additional patient populations and other hematologic malignancies. This includes approvals for CLL patients without the 17p deletion, as well as approvals for acute myeloid leukemia (AML) and small lymphocytic lymphoma.
In 2023, VENCLEXTA generated global revenue of USD 2.2 billion with growth rate of approximately 14% year on year basis. The G101V mutation in BCL-2 is frequently observed in patients who relapsed after being treated with venetoclax or confer resistance to venetoclax. Therefore, the development of next-generation BCL-2 inhibitors to overcome drug resistance is urgently needed. Sonrotoclax, a potent and selective BCL-2 inhibitor, demonstrates stronger cytotoxic activity in various hematologic cancer cells and more profound tumor growth inhibition in multiple hematologic tumor models than venetoclax. Notably, sonrotoclax effectively inhibits venetoclax-resistant BCL-2 variants, such as G101V.
Currently Sonrotoclax is a leading BCL-2 inhibitor in clinical advancement, presently in phase III trials as combination therapay with zanubrutini for the treatment of chronic lymphocytic leukemia and as a monotherapy for the treatment of various types of cancer in phase II. Several key BCL-2 inhibitors companies, including BeiGene, Zentalis pharmaceuticals, Ascentage pharma and others are involved in developing drugs for BCL-2 inhibitors for various indications such as relapsed or refractory chronic lymphocytic leukemia or small lymphocytic lymphoma, waldenstrom macroglobulinemia and others. Overall, this is an exciting new class of agents with great potential for development. The maturation of current studies over the next few years will lead to a better understanding of BCL-2 inhibitors and define their role in the therapy of cancer.
BCL-2 Inhibitor Drugs Uptake
This section focuses on the uptake rate of potential approved and emerging BCL-2 inhibitors expected to be launched in the BCL-2 inhibitors market during 2020–2034.
BCL-2 Inhibitors Clinical Trial Activities
The BCL-2 Inhibitors market report provides insights into different therapeutic candidates in phase III, phase II, and phase I. It also analyzes key BCL-2 Inhibitors companies involved in developing targeted therapeutics. The presence of numerous drugs under different stages is expected to generate immense opportunity for BCL-2i market growth over the forecasted period.
BCL-2 inhibitors Pipeline Development Activities
The BCL-2 Inhibitors market size report covers information on collaborations, acquisitions and mergers, licensing, and patent details for BCL-2 inhibitors emerging therapies.
BCL-2 Inhibitors Collaborations and Partnerships
The increasing strategic collaborations among major market players to enhance the growth of their pipeline products are anticipated to drive market expansion. For example, in October 2023, Ascentage pharma and AstraZeneca entered into clinical collaboration on the registrational phase III study of BCL-2 inhibitor Lisaftoclax (APG-2575) in combination with BTK inhibitor acalabrutinib in treatment-naïve patients with first-line CLL/SLL.
Lisaftoclax is a novel, orally administered small-molecule BCL-2 selective inhibitor being developed by Ascentage pharma to treat malignancies by selectively blocking the antiapoptotic protein BCL-2 and hence restoring the normal apoptosis process in cancer cells.
Latest KOL Views on BCL-2 Inhibitors
To keep up with current and future BCL-2 inhibitors market trends, we take industry experts’ opinions working in the domain through primary research to fill the data gaps and validate our secondary research. Industry experts were contacted for insights on BCL-2 inhibitors' evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, drug uptake, along challenges related to accessibility. DelveInsight’s analysts connected with 25+ KOLs to gather insights; however, interviews were conducted with 10+ KOLs in the 7MM. Their opinion helps understand and validate current and emerging therapy treatment patterns or BCL-2 inhibitors market trends. This will support the clients in understanding potential upcoming novel treatments by identifying the overall scenario of the BCL-2 inhibitors market and the unmet needs.
What KOLs are saying on BCL-2 Inhibitors Patient Trends? |
|
“The BCL-2 Inhibitors treatment of patients with relapsed/refractory acute myeloid leukemia remains challenging. The combination of enasidenib and venetoclax (BCL-2 inhibitor) represents a potential option for the group of patients with acute myeloid leukemia and IDH2 mutations.” |
Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving treatment landscape.
For More Information of the Report @ Relapsed/Refractory Acute Myeloid Leukemia Market
Key Updates on BCL-2 Inhibitors
Many Pharma BCL-2 Inhibitors Companies will be presenting the data of their BCL-2 inhibitors during the ASCO 2024 conference including Ascentage pharma and others. During the ASCO 2024 annual meeting, Ascentage pharma is expected to present the results of clinical trial NCT04260217, with the title “Updated efficacy and safety results of BCL-2 inhibitor lisaftoclax (APG-2575) alone or combined with ibrutinib or rituximab in patients (pts) with Waldenström macroglobulinemia (WM)” (Abstract # 7078).
The abstract list is not exhaustive, will be provided in the final report
BCL-2 Inhibitors Market Report Scope
- The BCL-2 Inhibitors market outlook report covers a segment of key events, an executive summary, and a descriptive overview of BCL-2 inhibitors, explaining their mechanism, and therapies (current and emerging).
- Comprehensive insight into the BCL-2 Inhibitors competitive landscape, and forecasts, the future growth potential of treatment rate, drug uptake, and drug information have been provided.
- Additionally, an all-inclusive account of the current and emerging therapies and the elaborative profiles of late-stage and prominent therapies will impact the current landscape.
- A detailed review of the BCL-2 inhibitors market, historical and forecasted market size, market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The BCL-2 Inhibitors market outlook report provides an edge while developing business strategies, by understanding trends, through SWOT analysis, expert insights/KOL views, and treatment preferences that help shape and drive the 7MM BCL-2 inhibitors market.
BCL-2 Inhibitors Market Report Insights
- BCL-2 Inhibitors Targeted Patient Pool
- Therapeutic Approaches
- BCL-2 Inhibitors Pipeline Analysis
- BCL-2 Inhibitors Market Size
- BCL-2 inhibitors Market Trends
- Existing and Future BCL-2 Inhibitors Market Opportunity
BCL-2 Inhibitors Market Report Key Strengths
- 10 years BCL-2 Inhibitors Market Forecast
- The 7MM Coverage
- Key Cross Competition
- BCL-2 inhibitors Drugs Uptake
- Key BCL-2 Inhibitors Market Forecast Assumptions
BCL-2 Inhibitors Market Report Assessment
- Current BCL-2 Inhibitors Treatment Market Practices
- BCL-2 Inhibitors Unmet Needs
- BCL-2 Inhibitors Pipeline Product Profiles
- BCL-2 Inhibitors Market Attractiveness
- Qualitative Analysis (SWOT)
Key Questions Answered in the BCL-2 Inhibitors Market Report
- What was the BCL-2 inhibitor market size, the BCL-2 Inhibitors market size by therapies, BCL-2 Inhibitors market share (%) distribution, and what would it look like in 2034? What are the contributing factors for this growth?
- Which BCL-2 inhibitors is going to be the largest contributor in 2034?
- Which is the most lucrative market for BCL-2 inhibitors?
- Which BCL-2 inhibitors type segment accounts for maximum BCL-2 inhibitor sales?
- What are the pricing variations among different geographies for approved therapies?
- How has the reimbursement landscape for BCL-2 inhibitors evolved since the first one was approved? Do patients have any access issues that are driven by reimbursement decisions?
- What are the risks, burdens, and unmet needs of treatment with BCL-2 inhibitors? What will be the growth opportunities across the 7MM for the patient population of BCL-2 inhibitors?
- What are the key factors hampering the growth of the BCL-2 inhibitors market?
- What are the indications for which recent novel therapies and technologies have been developed to overcome the limitations of existing treatments?
- What key designations have been granted to the therapies for BCL-2 inhibitors?
- What is the cost burden of approved BCL-2 inhibitors therapies on the patient?
- Patient acceptability in terms of preferred therapy options as per real-world scenarios?
- What are the country-specific accessibility issues of expensive, recently approved BCL-2 inhibitors therapies?
Reasons to Buy BCL-2 Inhibitors Market Report
- The BCL-2 Inhibitors Market Outlook Report will help develop business strategies by understanding the latest trends and changing dynamics driving the BCL-2 inhibitors Market.
- Understand the existing BCL-2 inhibitors market opportunities in varying geographies and the growth potential over the coming years.
- Distribution of historical and current BCL-2 inhibitors patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain) the United Kingdom, and Japan.
- Identifying strong upcoming BCL-2 inhibitors companies in the BCL-2 inhibitors market will help devise strategies to help get ahead of competitors.
- Detailed analysis and ranking of indication-wise current and emerging therapies under the conjoint analysis section to provide visibility around leading indications.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing BCL-2 inhibitors market so that the upcoming BCL-2 inhibitors companies can strengthen their development and launch strategy.