Chronic Spontaneous Urticaria Market Summary
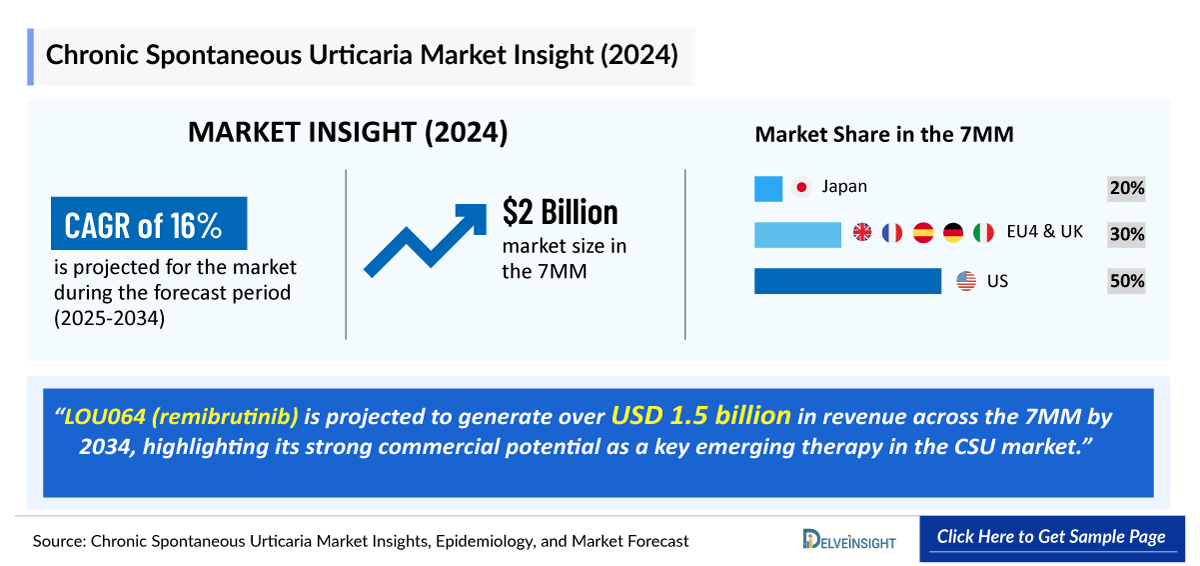
- The Chronic Spontaneous Urticaria market size in the 7MM was valued at approximately USD 2000 million in 2025 and is projected to reach USD 7,555 million by 2034 over the forecast period from 2025 to 2034.
- The Chronic Spontaneous Urticaria market is projected to grow at a CAGR of 15.9% by 2034 in leading countries (US, EU4, UK and Japan).
- The Chronic Spontaneous Urticaria market size in the US was approximately USD 1 billion in 2024 and will increase at a CAGR of 14% during the forecast period driven by the increasing awareness of the disease and the launch of the Chronic Spontaneous Urticaria emerging drug.
- The total market size of Chronic Spontaneous Urticaria in EU4 and the UK was calculated to be approximately USD 620 million in 2024, which was nearly 30% of the total market revenue for the 7MM.
Chronic Spontaneous Urticaria Market and Epidemiology Analysis
- According to DelveInsight’s estimates, in 2024, there were approximately 3.2 million diagnosed prevalent cases of Chronic Spontaneous Urticaria in the 7MM. Of these, the US accounted for nearly 18.5% of the cases, while the EU4 and the UK accounted for around 44.3% and Japan represented approximately 37.2% of the cases, respectively.
- The Chronic Spontaneous Urticaria treatment market is projected to see consistent growth, with a robust compound annual growth rate (CAGR) anticipated from 2025 to 2034. This expansion across the 7MM will be driven by the introduction of innovative therapies, barzolvolimab (CDX-0159), LOU064 (remibrutinib), rilzabrutinib, povorcitinib, and briquilimab, among others. Additionally, the rising prevalence of Chronic Spontaneous Urticaria, driven by factors such as an aging population, increased exposure to environmental irritants, underlying autoimmune dysregulation, and improved diagnostic awareness among healthcare professionals, will further support market growth.
- The recent approval of DUPIXENT (dupilumab) by the US FDA, along with its earlier approval in Japan by the MHLW, marks a notable shift in the treatment landscape, introducing a targeted therapies that is likely to drive market competition and influence the adoption.
- Despite the availability of treatments like antihistamines, omalizumab, and DUPIXENT, a significant proportion of patients with Chronic Spontaneous Urticaria continue to experience inadequate symptom control or relapses. Escalating antihistamine doses can lead to adverse effects such as sedation and anticholinergic toxicity, while omalizumab carries a risk of anaphylaxis. These limitations underscore the urgent need for safer and more effective therapies targeting alternative pathways, such as BTK inhibitors, which have shown encouraging results in early trials.
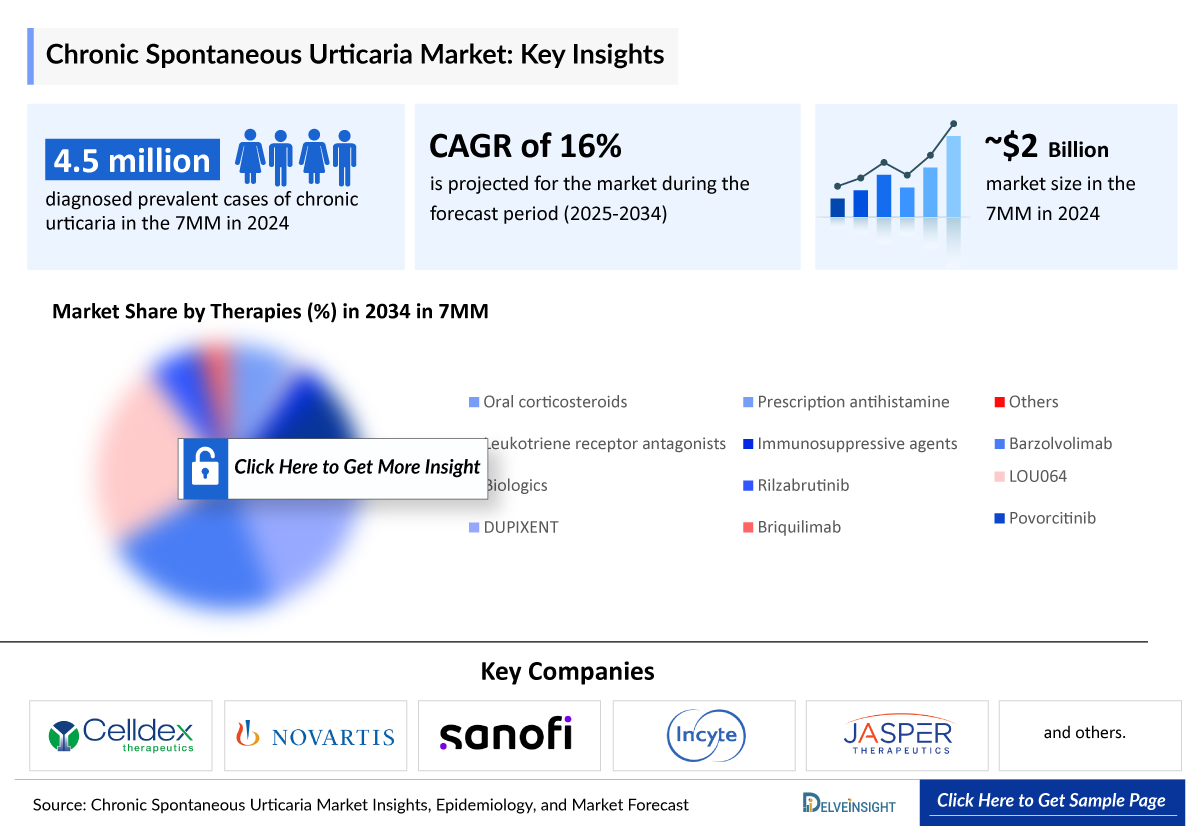
- Celldex Therapeutics, Novartis, Sanofi, Incyte, Jasper Therapeutics, and Evommune, among others are progressing their assets through various Chronic Spontaneous Urticaria clinical trials phases, driving innovation in the chronic pruritus market and creating significant growth opportunities.
- Among the emerging therapies, regulatory submissions for Novartis’ remibrutinib in adults are planned for the first half of 2025 in both the US and Europe, with US FDA approval for Chronic Spontaneous Urticaria expected in the second half of 2025.
DelveInsight’s “Chronic Spontaneous Urticaria (Chronic Spontaneous Urticaria) Market Insights, Epidemiology, and Market Forecast 2034” report delivers an in-depth understanding of Chronic Spontaneous Urticaria, historical and forecasted epidemiology, as well as the Chronic Spontaneous Urticaria therapeutics market trends in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
The Chronic Spontaneous Urticaria therapeutics market report provides current treatment practices, emerging Chronic Spontaneous Urticaria drugs, Chronic Spontaneous Urticaria market share of individual therapies, and current and forecasted 7MM Chronic Spontaneous Urticaria market size from 2020 to 2034. The report also covers Chronic Spontaneous Urticaria treatment practices/algorithms and unmet medical needs to curate the best opportunities and assess the Chronic Spontaneous Urticaria therapeutics market potential.
|
Study Period |
2020–2034 |
|
Forecast Period |
2025–2034 |
|
Geographies Covered |
US, EU4 (Germany, France, Italy, and Spain) and the UK, and Japan |
|
Chronic Spontaneous Urticaria Epidemiology |
|
|
Chronic Spontaneous Urticaria Market |
|
|
Chronic Spontaneous Urticaria Market Analysis |
|
|
Chronic Spontaneous Urticaria Market companies |
|
|
Chronic Spontaneous Urticaria Future opportunity |
A significant growth driver in Chronic Spontaneous Urticaria involves leveraging the influx of targeted therapies, such as barzolvolimab (CDX-0159), LOU064 (remibrutinib), rilzabrutinib, povorcitinib, and briquilimab, to address the sizeable unmet need among patients inadequately controlled on antihistamines or omalizumab; these novel agents can offer safer, more durable symptom control, capture market share as remibrutinib and similar molecules obtain regulatory approvals (for example, remibrutinib’s anticipated US FDA approval in 2H 2025), and position themselves competitively against DUPIXENT’s entry, thereby driving sustained market growth in the 7MM as prevalence rises and diagnostic awareness improves. |
Key Factors Driving the Chronic Spontaneous Urticaria Market
Growing Chronic Spontaneous Urticaria Prevalence
According to DelveInsight’s estimates, the total diagnosed prevalent cases of chronic urticaria in the 7MM were approximately 4.5 million in 2024, with these numbers anticipated to increase by 2034.
DUPIXENT’s Monopoly in the CSU Market
The recent approval of DUPIXENT (dupilumab) by the US FDA, along with its earlier approval in Japan by the MHLW, marks a notable shift in the treatment landscape, introducing targeted therapies that are likely to drive market competition and influence the adoption.
Growing CSU Clinical Trial Activity
The CSU treatment pipeline is increasingly competitive, featuring several promising candidates at different stages of development, including barzolvolimab (CDX-0159), remibrutinib (LOU064), rilzabrutinib, povorcitinib, and briquilimab, among others.
Chronic Spontaneous Urticaria Understanding and Treatment Algorithm
Urticaria is a common and heterogeneous inflammatory skin disorder with or without associated angioedema. It presents with wheals, angioedema, or both due to activation and degranulation of skin mast cells, followed by the release of histamine and other mediators leading to sensory nerve activation, vasodilatation, plasma extravasation, and cellular recruitment. It is classified as acute or chronic, depending on whether the onset of episodes lasts for less or >6 weeks, respectively.
Chronic urticaria is spontaneous or inducible, lasts >6 weeks, and persists for >1 year. It impacts the quality of life and is linked to psychiatric comorbidities and high healthcare costs, often causing huge socio-economic distress for the patients. In contrast to Chronic Spontaneous Urticaria, where the cause is unknown, chronic inducible urticaria has definite and subtype-specific triggers that induce signs and symptoms.
A mast cell-driven disease is characterized by recurrent itchy wheals (hives) that may accompany angioedema, due to activation and degranulation of skin mast cells, followed by the release of histamine and other mediators. The typical lesion is a pale-to-red, well-demarcated papule or plaque. Lesions may be round, oval, annular, arcuate, serpiginous, or generalized. They resolve without post-inflammatory pigmentary changes or scaling. Women are twice as likely as men to be diagnosed with the disease, and most people first develop symptoms between 20 and 40 years.
The etiology of Chronic Spontaneous Urticaria is yet to be fully established. The exact cause is often unknown, but it may be due to autoimmune reactions wherein the immune system mistakenly targets healthy cells in the skin. Other potential triggers include medications, infections, insect bites, stress, and temperature changes. The prognosis in Chronic Spontaneous Urticaria depends on the comorbid disease causing the urticaria and the patient’s response to therapy. The autoimmune pathogenesis of chronic urticaria, including recent data, suggests that Chronic Spontaneous Urticaria may involve contributions from both immunoglobulin G (IgG)-specific and immunoglobulin E (IgE)-specific autoantibodies against a vast array of antigens that can span beyond those found on the surface of mast cells and basophils.
Chronic Spontaneous Urticaria diagnosis
The diagnosis is based on a physical examination and medical history. Additional tests are performed to rule out underlying causes or to identify triggers, such as blood tests, allergy tests, or skin biopsies. Screening tests for thyroid function and antithyroid peroxidase and antithyroglobulin antibodies are recommended. Positive autologous serum skin test (ASST) and in vitro testing of the patient’s serum for the anti-FCeRIa or the anti-IgE autoantibodies by basophil histamine release assay (BHRA) is also recommended.
Some tools have been developed to assess disease activity (e.g., urticaria activity score), disease control (e.g., urticaria control test), and impacts on quality of life (e.g., chronic urticaria quality of life index). Baseline assessments should be performed to help guide treatment decisions and monitor progress.
Further details related to country-based variations are provided in the report…
Chronic Spontaneous Urticaria treatment
Chronic Spontaneous Urticaria management follows a stepwise approach aimed at complete symptom control and quality of life improvement. First-line treatment consists of second-generation H1-antihistamines due to their safety, while first-generation agents are discouraged. For non-responders, up-dosing H1-antihistamines up to fourfold is advised, with optional H2-antihistamines, though evidence for the latter is inconsistent.
Omalizumab, an anti-IgE monoclonal antibody, is the preferred second-line option for antihistamine-refractory Chronic Spontaneous Urticaria and is effective, well-tolerated, and suitable for long-term use. Cyclosporine may be added in severe, treatment-resistant cases but is limited by safety concerns. Short courses of systemic corticosteroids can manage acute flares, but long-term use is discouraged. Leukotriene receptor antagonists, particularly montelukast, offer modest adjunctive benefit.
Treatment should be reassessed every 3-6 months due to the fluctuating nature of Chronic Spontaneous Urticaria. Despite guidelines, non-recommended therapies, such as sedating antihistamines and prolonged corticosteroids, remain in use in real-world practice.
Chronic Spontaneous Urticaria Epidemiology
As the Chronic Spontaneous Urticaria market is derived using a patient-based model, the Chronic Spontaneous Urticaria epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented total diagnosed prevalent cases of chronic urticaria, type-specific cases of chronic urticaria, gender-specific cases of Chronic Spontaneous Urticaria, age-specific cases of Chronic Spontaneous Urticaria, and severity-specific cases of Chronic Spontaneous Urticaria in the 7MM covering the United States, EU4 countries (Germany, France, Italy, and Spain) and the United Kingdom, and Japan from 2020 to 2034.
Key Findings from Chronic Spontaneous Urticaria Epidemiological Analyses and Forecast
- According to DelveInsight’s estimates, the total diagnosed prevalent cases of chronic urticaria in the 7MM were approximately 4.5 million in 2024, with these numbers anticipated to increase by 2034.
- Among the 7MM, the US accounted for nearly 19% of the total diagnosed prevalent cases of chronic urticaria, with around 885 thousand cases in 2024. These cases are expected to change during the forecast period (2025–2034). The EU4 and the UK accounted for approximately 39% of the total cases, while Japan accounted for around 42% in 2024.
- DelveInsight’s estimates indicate that type-specific diagnosed prevalent cases of chronic urticaria, including Chronic Spontaneous Urticaria and Chronic Inducible Urticaria, were nearly 3.2 million and 1.5 million cases, respectively, in 2024 in the 7MM.
- The US accounted for approximately 600 thousand diagnosed prevalent cases of Chronic Spontaneous Urticaria in 2024, and these cases are projected to increase by 2034.
- In 2024, among the EU4 and the UK, Germany reported the highest number of diagnosed prevalent cases of Chronic Spontaneous Urticaria with approximately 375 thousand cases, while Spain reported the lowest.
- Japan accounted for nearly 37% of the total diagnosed prevalent Chronic Spontaneous Urticaria cases across the 7MM in 2024.
- DelveInsight estimates that Chronic Spontaneous Urticaria shows a significant female predominance compared to males. Among the gender-specific diagnosed prevalent cases of Chronic Spontaneous Urticaria, the US accounted for approximately 27% males and 73% females in 2024.
- According to DelveInsight’s age-specific prevalence estimates, in the EU4 and the UK, the age group =60 years represented the largest number of diagnosed Chronic Spontaneous Urticaria cases in 2024, with nearly 445 thousand cases, followed by the 50–59 years age group, with around 235 thousand cases.
- DelveInsight’s severity-specific prevalence estimates for Japan in 2024 reported around 215 thousand, 455 thousand, and 515 thousand cases of mild, moderate, and severe Chronic Spontaneous Urticaria, respectively, with these numbers expected to change in the forecast period (2025–2034).
Chronic Spontaneous Urticaria Epidemiology Segmentation
- Total Diagnosed Prevalent Cases of Chronic Urticaria
- Type-specific Cases of Chronic Urticaria
- Gender-specific Cases of Chronic Spontaneous Urticaria
- Age-specific Cases of Chronic Spontaneous Urticaria
- Severity-specific Cases of Chronic Spontaneous Urticaria
Chronic Spontaneous Urticaria Market Recent Developments and Breakthroughs
- In April 2025, Regeneron Pharmaceuticals and Sanofi announced that the FDA approved Dupixent® (dupilumab) for treating chronic spontaneous urticaria (CSU) in adults and adolescents aged 12 years and older who remain symptomatic despite H1 antihistamine treatment. The approval provides an additional option for patients suffering from this debilitating condition.
Chronic Spontaneous Urticaria Drug Analysis
The drug chapter segment of the Chronic Spontaneous Urticaria report encloses a detailed analysis of Chronic Spontaneous Urticaria marketed Chronic Spontaneous Urticaria market drugs and mid to late-stage (Phase III and Phase II) pipeline Chronic Spontaneous Urticaria drugs. It also helps understand the Chronic Spontaneous Urticaria clinical trials details, expressive pharmacological action, agreements and collaborations, approval and patent details, advantages and disadvantages of each included drug and the latest news and press releases.
Marketed Chronic Spontaneous Urticaria Drugs
DUPIXENT (dupilumab): Sanofi/Regeneron Pharmaceuticals
DUPIXENT (dupilumab) is a human monoclonal IgG4 antibody that inhibits interleukin-4 (IL-4) and interleukin-13 (IL-13) signaling by specifically binding to the IL-4Ra subunit shared by the IL-4 and IL-13 receptor complexes. Dupilumab inhibits IL-4 signaling via the ‘Type I’ receptor and both IL-4 and IL-13 signaling through the ‘Type II receptor.’ DUPIXENT is approved for multiple indications, including atopic dermatitis, asthma, and chronic rhinosinusitis with nasal polyposis, eosinophilic esophagitis, and prurigo nodularis.
DUPIXENT was first approved for the treatment of Chronic Spontaneous Urticaria in February 2024 in Japan, becoming the first country globally to authorize its use for this indication. The approval was based on results from a Phase III trial that demonstrated a significant reduction in itch compared to placebo. Subsequently, in April 2025, the US approved DUPIXENT as the first new targeted therapy for Chronic Spontaneous Urticaria in over a decade.
|
Drug |
MoA |
RoA |
Company |
|
DUPIXENT (dupilumab) |
IL-4 and IL-13 inhibitor |
SC |
Sanofi/Regeneron |
Emerging Chronic Spontaneous Urticaria Drugs
Barzolvolimab (CDX-0159): Celldex Therapeutics
Barzolvolimab is a humanized monoclonal antibody that selectively targets KIT, a receptor tyrosine kinase critical for mast cell survival, activation, and recruitment to tissues. Since mast cells play a key role in driving inflammation in Chronic Spontaneous Urticaria, inhibiting KIT offers a promising therapeutic approach. Barzolvolimab is currently being investigated in a Phase III trial for Chronic Spontaneous Urticaria.
Celldex Therapeutics continues to build momentum for barzolvolimab in chronic urticaria. At the American Academy of Allergy, Asthma & Immunology (AAAAI) Annual Meeting in March 2025, the company presented promising Phase II data demonstrating improvements in disease control and quality of life.
In May 2025, Celldex announced that 76-week results, including a 24-week off-treatment follow-up, from its Phase II study in Chronic Spontaneous Urticaria were accepted for a late-breaking oral presentation at the European Academy of Allergy and Clinical Immunology (EAACI) Congress in June 2025.
LOU064 (remibrutinib): Novartis
Remibrutinib is an investigational, highly selective, covalent oral Bruton’s Tyrosine Kinase (BTK) inhibitor that disrupts the BTK signaling pathway, preventing histamine release responsible for itching and swelling in Chronic Spontaneous Urticaria. When combined with standard-dose antihistamines, it offers a dual mechanism of action: remibrutinib blocks histamine release, while antihistamines block histamine receptors, together effectively reducing symptoms.
In February 2025, Novartis presented findings from 17 abstracts at the 2025 AAAAI and World Allergy Organization (WAO) Joint Congress and the American Academy of Dermatology (AAD) Annual Meeting, including long-term data on urticaria control, sleep, and activity outcomes from the Phase III REMIX-1 and REMIX-2 trials evaluating investigational remibrutinib for the treatment of Chronic Spontaneous Urticaria.
In the pivotal Phase III REMIX-1 and REMIX-2 trials, remibrutinib achieved all primary endpoints in patients with Chronic Spontaneous Urticaria who remained symptomatic despite second-generation H1-antihistamines, demonstrating rapid and sustained symptom relief through Week 52.
Regulatory submissions for remibrutinib in adults are planned for the first half of 2025 in the US, utilizing a Priority Review Voucher (PRV), and in Europe, with US FDA approval for Chronic Spontaneous Urticaria anticipated in the second half of 2025. Additionally, the drug is being evaluated in Phase III trials for pediatric patients.
Rilzabrutinib: Sanofi
Rilzabrutinib is an oral, reversible, covalent BTK inhibitor with the potential to become a first- or best-in-class therapy for several immune-mediated diseases. Targeting BTK, which is expressed in B cells and mast cells and plays a key role in immune responses, rilzabrutinib leverages Sanofi’s TAILORED COVALENCY technology to achieve selective inhibition while minimizing off-target side effects.
In 2024, the Phase II RILEChronic Spontaneous Urticaria trial in Chronic Spontaneous Urticaria patients unresponsive to H1 antihistamines reported positive results, paving the way for further evaluation in Phase III studies
In February 2024, Sanofi presented positive results from the Phase II RILE Chronic Spontaneous Urticaria study at the AAAAI Annual Meeting, demonstrating that rilzabrutinib significantly improved itch, hives, and overall urticaria symptoms in adults with moderate-to-severe Chronic Spontaneous Urticaria who remained symptomatic despite H1 antihistamine treatment.
|
Drug |
MoA |
RoA |
Company |
Phase |
|
Barzolvolimab (CDX-0159) |
KIT inhibitor |
SC |
Celldex Therapeutics |
III |
|
LOU064 (remibrutinib) |
BTK inhibitor |
Oral |
Novartis |
III |
|
Rilzabrutinib |
Reversible covalent BTK inhibitor |
Oral |
Sanofi |
II |
Chronic Spontaneous Urticaria Drug Class Analysis
Chronic Spontaneous Urticaria is a chronic condition characterized by the recurrent appearance of hives or wheals on the skin. It is a disturbing allergic condition of the skin, where symptoms persist for more than 6 weeks. The diagnosis is based on a physical examination and medical history. Additional tests are performed to rule out underlying causes or to identify triggers, such as blood tests, allergy tests, or skin biopsies. Treating Chronic Spontaneous Urticaria is challenging, and the therapeutic goal is a reduction in disease activity, complete symptom control, and improvement in QoL. The current treatment regime aims to alleviate symptoms and prevent their recurrence. The treatment pattern typically involves a stepwise approach, starting with first-line treatments and progressing to more advanced options if necessary.
Standard-dose of second-generation H1- antihistamines are the primary class of medications used to treat Chronic Spontaneous Urticaria. They work by blocking histamine H1 receptors in the body, alleviating the symptoms of itching, redness, and swelling associated with Chronic Spontaneous Urticaria. Treatment is generally initiated with nonsedating antihistamines in the daytime and sedating antihistamines at night. H2 antihistamines are often combined with H1 to achieve better symptom control in Chronic Spontaneous Urticaria and are added if individuals complain of indigestion or acidity. Omalizumab, an anti-IgE monoclonal antibody, is the next-in-line therapy to be given as an add-on to improve treatment efficacy. Omalizumab is approved for Chronic Spontaneous Urticaria patients age 12 years and older who remain symptomatic despite H1-antihistamine treatment. In Chronic Spontaneous Urticaria, omalizumab prevents wheal and angioedema development and improves the quality of life.
Patients with urticaria who do not show sufficient benefit from treatment with omalizumab are treated with cyclosporine 3.5–5 mg/kg per day. Cyclosporine is immunosuppressive and has a moderate, direct effect on mast cell mediator release. The use of leukotriene receptor antagonists like montelukast, zafirlukast, zileuton, etc., has also been assessed in various trials. In general, the evidence for the efficacy of leukotriene receptor antagonists in urticaria is low, but best for montelukast.
The guidelines also state that for acute urticaria and exacerbations of Chronic Spontaneous Urticaria, a short course of oral corticosteroids helps reduce disease duration and activity.
Continued in report…
Chronic Spontaneous Urticaria Market Outlook
Chronic Spontaneous Urticaria is a disturbing allergic condition of the skin where symptoms persist for more than 6 weeks. A mast cell-driven disease is characterized by recurrent itchy wheals (hives) that may accompany angioedema. It often causes huge socio-economic distress for the patients, significantly impacting their quality of life.
Treating Chronic Spontaneous Urticaria is challenging, and the therapeutic goal is a reduction in disease activity, complete symptom control, and improvement in QoL. The current treatment regime aims to alleviate symptoms and prevent their recurrence. The treatment pattern typically involves a stepwise approach, starting with first-line treatments and progressing to more advanced options if necessary.
Treatment patterns vary depending on the individual and their response to different medications. Various factors, such as the severity of symptoms, treatment response, and any underlying conditions, are to be considered for the most appropriate treatment plan.
The international EAACI/GA²LEN/EuroGuiDerm/APAAACI guideline recommends second-generation H1-antihistamine as first-line treatment for all types of urticaria. The up-dosing of second-generation H1- antihistamine up to fourfold in patients with chronic urticaria unresponsive to a standard dose is recommended as a second-line treatment before other treatments are considered. The guidelines further recommend the approved monoclonal antibody, omalizumab, for treating patients with chronic urticaria unresponsive to high-dose antihistamines. Cyclosporine is used off-label and is recommended only for patients with severe disease, refractory to any dose of antihistamine and omalizumab in combination.
DUPIXENT, an IL-4 receptor alpha antagonist, represents a significant advancement in the treatment of Chronic Spontaneous Urticaria, offering a targeted option for adults and adolescents aged 12 years and older who remain symptomatic despite H1-antihistamine therapy. Unlike conventional treatments, DUPIXENT specifically targets type 2 inflammation, a key driver in Chronic Spontaneous Urticaria, providing a more precise and effective approach. Approved in both the US and Japan, it stands out as the only newly approved biologic in over a decade for Chronic Spontaneous Urticaria, with no indication for other forms of urticaria.
The current Chronic Spontaneous Urticaria market has been segmented into different commonly used therapeutic classes based on the prevailing treatment pattern across the 7MM, which presents minor variations in the overall prescription pattern. Oral corticosteroids, prescription antihistamines, leukotriene receptor antagonists, immunosuppressive agents, omalizumab, DUPIXENT and others are the major Chronic Spontaneous Urticaria market drugs covered in the forecast model.
Key Chronic Spontaneous Urticaria companies Celldex Therapeutics’ Barzolvolimab, Novartis’ LOU064 (remibrutinib), and Sanofi's Rilzabrutinib (SAR444671), among others, are evaluating their lead candidates in different stages of Chronic Spontaneous Urticaria clinical trials. They aim to investigate their products to treat Chronic Spontaneous Urticaria.
- The Chronic Spontaneous Urticaria market in the 7MM was valued at approximately USD 2000 million in 2025 and is projected to reach USD 7,555 million by 2034 at a CAGR of 15.9% over the forecast period from 2025 to 2034.
- The Chronic Spontaneous Urticaria market size in the US was approximately USD 1 billion in 2024 and will increase at a CAGR of 14% during the forecast period driven by the increasing awareness of the disease and the launch of the Chronic Spontaneous Urticaria emerging drug.
- The total market size of Chronic Spontaneous Urticaria in EU4 and the UK was calculated to be approximately USD 620 million in 2024, which was nearly 30% of the total market revenue for the 7MM.
- Among EU4 and the UK, Germany accounted for the highest Chronic Spontaneous Urticaria market with approximately USD 165 million in 2024, while Spain accounted for the lowest market with nearly USD 80 million in 2024.
- In 2024, biologics dominated the Chronic Spontaneous Urticaria treatment landscape across the 7MM, capturing the largest Chronic Spontaneous Urticaria market share with revenue of approximately USD 1 billion. This was followed by prescription antihistamines at around USD 490 million and immunosuppressive agents at approximately USD 215 million, reflecting a clear preference for targeted biologic therapies in Chronic Spontaneous Urticaria clinical trials.
- In 2024, the Chronic Spontaneous Urticaria market size in Japan was nearly USD 335 million which is expected to change by 2034.
- LOU064 (remibrutinib) is projected to generate over USD 1.5 billion in revenue across the 7MM by 2034, highlighting its strong commercial potential as a key emerging therapy in the Chronic Spontaneous Urticaria market.
Chronic Spontaneous Urticaria Drugs Uptake
This section focuses on the uptake rate of potential Chronic Spontaneous Urticaria drugs expected to be launched in the Chronic Spontaneous Urticaria market during 2020-2034. For example, Novartis’ LOU064 (remibrutinib), a BTK inhibitor, is expected to enter the US market by 2026 and is projected to have a medium uptake during the forecast period.
Further detailed analysis of emerging therapies drug uptake in the report…
Chronic Spontaneous Urticaria Pipeline Development Activities
The Chronic Spontaneous Urticaria pipeline report provides insights into different Chronic Spontaneous Urticaria clinical trials within Phase III, Phase II, and Phase I. It also analyzes key Chronic Spontaneous Urticaria companies involved in developing targeted therapeutics.
Pipeline development activities
The report covers information on collaborations, acquisitions and mergers, licensing, and patent details for Chronic Spontaneous Urticaria emerging therapies.
Latest KOL Views on Chronic Spontaneous Urticaria
To keep up with current market trends, we take KOLs and SMEs’ opinions working in the domain through primary research to fill the data gaps and validate our secondary research. Industry Experts contacted for insights on chronic pruritus evolving treatment landscape, patient reliance on conventional Chronic Spontaneous Urticaria therapies, patient therapy switching acceptability, and drug uptake, along with challenges related to accessibility, including Medical/scientific writers, Medical Professionals, Professors, Directors, and Others.
What KOLs are saying on Chronic Spontaneous Urticaria Patient Trends?
DelveInsight’s analysts connected with 50+ KOLs to gather insights; however, interviews were conducted with 15+ KOLs in the 7MM. Centers like the University of California, US, Johns Hopkins University School of Medicine, US, Oregon Health and Science University, US, University of Chicago, US, Institute of Allergology, Charité-Universitätsmedizin, Germany, University Hospital of Tours, France, Italian College of General Practitioners and Primary Care, Italy, Association of Chronic Urticaria Affected (AAUC), Spain, St James’s University Hospital, Leeds, UK, Department of Dermatology, Hiroshima Citizens Hospital, Japan, and Osaka Medical and Pharmaceutical University, Japan, among others, were contacted. Their opinion helps understand and validate current and emerging Chronic Spontaneous Urticaria therapy treatment patterns or chronic pruritus market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
Chronic Spontaneous Urticaria Physician’s View
As per the KOLs from the US, Chronic Spontaneous Urticaria is driven by complex skin inflammation involving mast cells, eosinophils, and basophils, leading to persistent wheals and angioedema that impair quality of life. While targeted therapies like omalizumab and dupilumab offer symptom relief, they do not address the underlying immune imbalance. Deeper insights into Chronic Spontaneous Urticaria pathogenesis are essential to enable curative, mechanism-based treatments.
As per the KOLs from France, the second-generation H1-antihistamines are used as the 1L of treatment for Chronic Spontaneous Urticaria. Increasing the dose of H1-antihistamines is recommended if the condition is not adequately controlled. Moreover, additional medications, primarily omalizumab and cyclosporine, added to treatment with antihistamines and medicines, are part of the 2L and 3L treatment therapy, respectively.
As per the KOLs from Japan, the absence of reliable predictive biomarkers in Chronic Spontaneous Urticaria limits clinicians’ ability to tailor treatment, often resulting in prolonged trial-and-error with antihistamines before escalating to biologics. While markers like CRP and D-dimer correlate with disease activity, their low predictive value hinders early intervention, delaying effective control in a substantial subset of patients. Bridging this gap is critical to advancing personalized care in Chronic Spontaneous Urticaria.
Chronic Spontaneous Urticaria Report Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the Analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving treatment landscape.
Attribute analysis analyzes multiple emerging Chronic Spontaneous Urticaria therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy.
To analyze the effectiveness of these Chronic Spontaneous Urticaria therapies, have calculated their attributed analysis by giving them scores based on their ability to improve atrial and ventricular dimension/function and ability to regulate heart rate.
Further, the therapies’ safety is evaluated wherein the adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials, which directly affects the safety of the molecule in the upcoming trials. It sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the route of administration, order of entry and designation, probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging Chronic Spontaneous Urticaria therapies are decided.
Chronic Spontaneous Urticaria Market Access and Reimbursement
DUPIXENT My Way Copay Card
The DUPIXENT My Way Co-pay Card may help eligible patients cover the out-of-pocket cost of DUPIXENT. With the DUPIXENT My Way Co-pay Card, eligible, commercially insured patients may pay as little as USD 0 co-pay per fill of DUPIXENT.
Eligibility for the DUPIXENT My Way Co-pay Card requires:
- Having commercial insurance, such as through health insurance exchanges, a federal employee plan, or a state employee plan.
- Residence within the 50 US states, the District of Columbia, Puerto Rico, Guam, or the US Virgin Islands.
- A prescription for DUPIXENT for an indication approved by the US FDA.
Further details will be provided in the report.
The report provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenarios, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Scope of the Chronic Spontaneous Urticaria Treatment Market Report
- The Chronic Spontaneous Urticaria therapeutics market report covers a segment of key events, an executive summary, and a descriptive overview of Chronic Spontaneous Urticaria, explaining its causes, signs and symptoms, pathogenesis, and currently available Chronic Spontaneous Urticaria therapies.
- Comprehensive insight into the epidemiology segments and forecasts, the future Chronic Spontaneous Urticaria market growth potential of diagnosis rate, disease progression, and treatment guidelines have been provided.
- Additionally, an all-inclusive account of the current and emerging Chronic Spontaneous Urticaria therapies and the elaborative profiles of late-stage and prominent therapies will impact the current treatment landscape.
- A detailed review of the Chronic Spontaneous Urticaria market, historical and forecasted market size, market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The report provides an edge while developing business strategies by understanding trends through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help shape and drive the 7MM Chronic Spontaneous Urticaria market.
Chronic Spontaneous Urticaria Treatment Market Report Insights
- Chronic Spontaneous Urticaria Patient Population
- Chronic Spontaneous Urticaria Therapeutic Approaches
- Chronic Spontaneous Urticaria Pipeline Analysis
- Chronic Spontaneous Urticaria Market Size and Trends
- Existing and Future Market Opportunity
Chronic Spontaneous Urticaria Treatment Market Report Key Strengths
- 10 years Forecast
- The 7MM Coverage
- Chronic Spontaneous Urticaria Epidemiology Segmentation
- Key Cross Competition
- Attribute analysis
- Chronic Spontaneous Urticaria market Drugs Uptake and Key Market Forecast Assumptions
Chronic Spontaneous Urticaria Treatment Market Report Assessment
- Current Chronic Spontaneous Urticaria Treatment Practices
- Chronic Spontaneous Urticaria Unmet Needs
- Chronic Spontaneous Urticaria Pipeline Product Profiles
- Chronic Spontaneous Urticaria Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
- Chronic Spontaneous Urticaria Market Drivers
- Chronic Spontaneous Urticaria Market Barriers
Key Questions Answered In The Chronic Spontaneous Urticaria Market Report:
Chronic Spontaneous Urticaria Market Insights
- What was the total market size of Chronic Spontaneous Urticaria, the market size of Chronic Spontaneous Urticaria by therapies, and market share (%) distribution in 2020, and what would it look like by 2034? What are the contributing factors for this growth?
- How will LOU064 (remibrutinib) affect the treatment paradigm of Chronic Spontaneous Urticaria?
- How will DUPIXENT compete with similar-class products and off-label therapies?
- Which drug is going to be the largest contributor by 2034?
- What are the pricing variations among different geographies for approved and marketed therapies?
- How would future opportunities affect the market dynamics and subsequent analysis of the associated trends
Chronic Spontaneous Urticaria Epidemiology Insights
- What are the disease risks, burdens, and unmet needs of Chronic Spontaneous Urticaria? What will be the growth opportunities across the 7MM with respect to the patient population pertaining to Chronic Spontaneous Urticaria?
- What is the historical and forecasted Chronic Spontaneous Urticaria patient pool in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan?
- Out of the countries mentioned above, which country would have the highest diagnosed prevalent Chronic Spontaneous Urticaria population during the forecast period (2025–2034)?
- What factors are contributing to the growth of Chronic Spontaneous Urticaria cases?
Current Treatment Scenario, Chronic Spontaneous Urticaria Marketed Therapies, and Chronic Spontaneous Urticaria Emerging Therapies
- What are the current options for the treatment of Chronic Spontaneous Urticaria? What are the current Chronic Spontaneous Urticaria clinical trials and treatment guidelines for treating Chronic Spontaneous Urticaria?
- How many Chronic Spontaneous Urticaria market companies are developing Chronic Spontaneous Urticaria therapies for the treatment of Chronic Spontaneous Urticaria?
- How many emerging Chronic Spontaneous Urticaria therapies are in the mid-stage and late stage of development for treating Chronic Spontaneous Urticaria?
- What are the recent novel Chronic Spontaneous Urticaria therapies, targets, mechanisms of action, and technologies developed to overcome the limitations of existing therapies?
- What is the cost burden of current treatment on the patient?
- Patient acceptability in terms of preferred treatment options as per real-world scenarios?
- What are the accessibility issues of approved Chronic Spontaneous Urticaria therapy in the US?
- What is the 7MM historical and forecasted market of Chronic Spontaneous Urticaria?
Reasons to Buy our Chronic Spontaneous Urticaria Market Forecast Report
- The report will help develop business strategies by understanding the latest trends and changing treatment dynamics driving the Chronic Spontaneous Urticaria market.
- Insights on patient burden/disease prevalence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years.
- Understand the existing market opportunities in varying geographies and the growth potential over the coming years.
- The distribution of historical and current patient share is based on real-world prescription data in the US, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
- Identifying upcoming solid Chronic Spontaneous Urticaria companies in the market will help devise strategies to help get ahead of competitors.
- Detailed analysis and ranking of class-wise potential current and emerging Chronic Spontaneous Urticaria therapies under the conjoint analysis section to provide visibility around leading classes.
- Highlights of Access and Reimbursement policies for Chronic Spontaneous Urticaria, barriers to accessibility of approved Chronic Spontaneous Urticaria therapy, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing market so that the upcoming Chronic Spontaneous Urticaria companies can strengthen their development and launch strategy.
Get interesting insights through our blog section @ DelveInsight Blogs

.png)