Prurigo Nodularis Market Summary
-
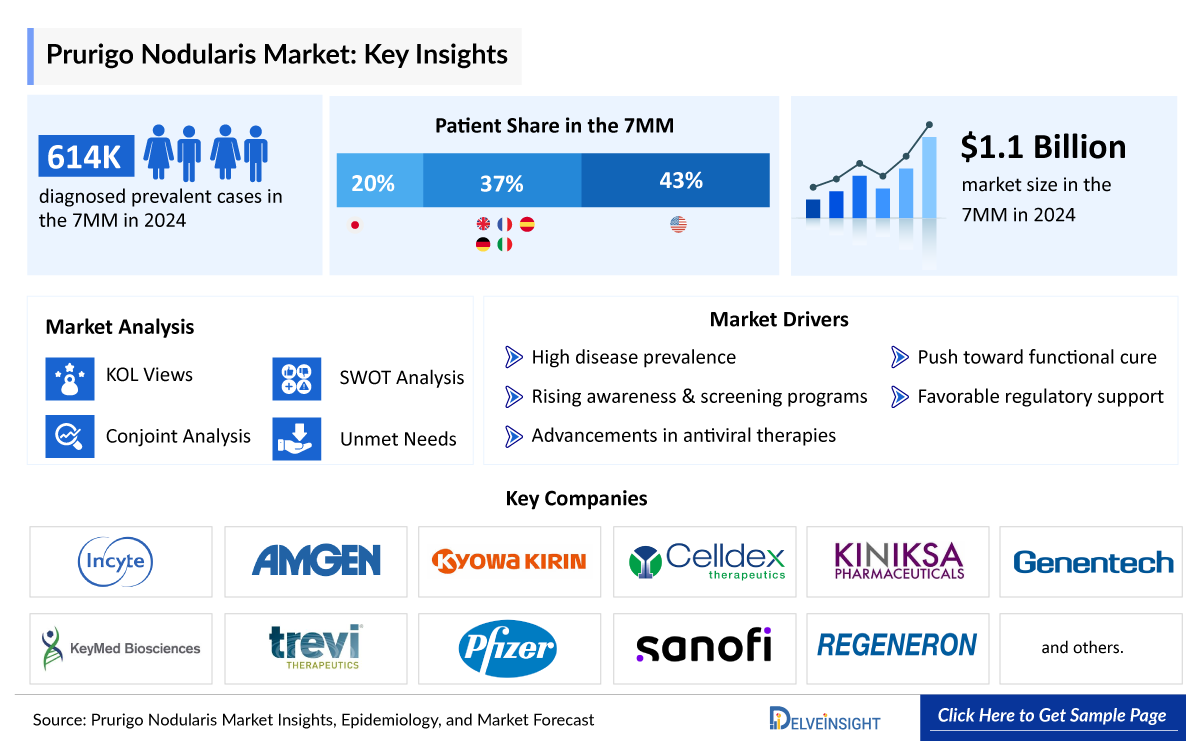
The Prurigo Nodularis market in the 7MM was valued at approximately USD 1,488 million in 2025 and is projected to reach USD 4,433 million by 2034 over the forecast period from 2025 to 2034.
-
The Prurigo Nodularis market is projected to grow at a CAGR of 12.90% by 2034 in leading countries (US, EU4, UK and Japan).
-
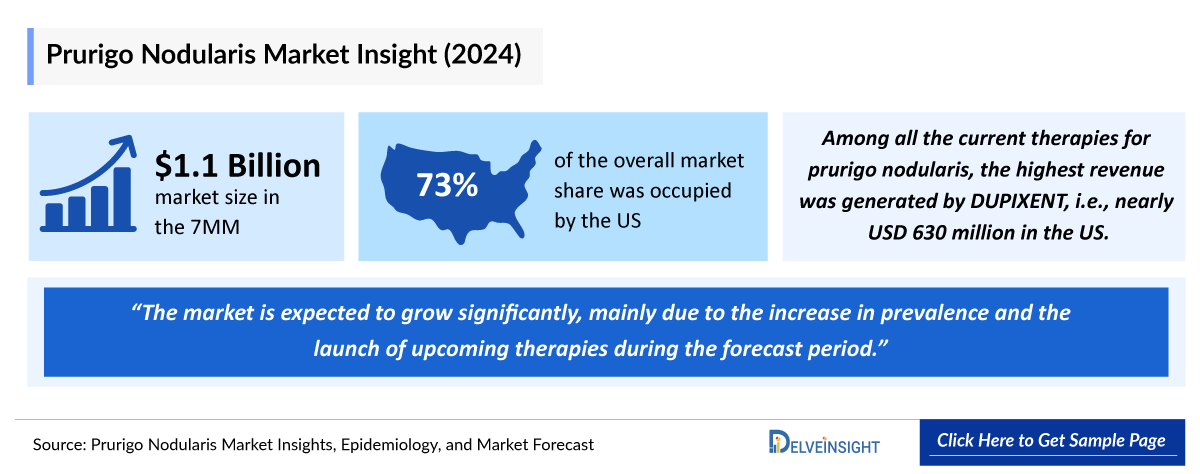
Among the 7MM, the United States accounted for the largest market size of prurigo nodularis, i.e., approximately 75% of the overall market in 2024.
- Among the 7MM, the US accounted for the largest market size of prurigo nodularis. i.e., USD ~810 million in 2024.
Prurigo Nodularis Market and Epidemiology Analysis
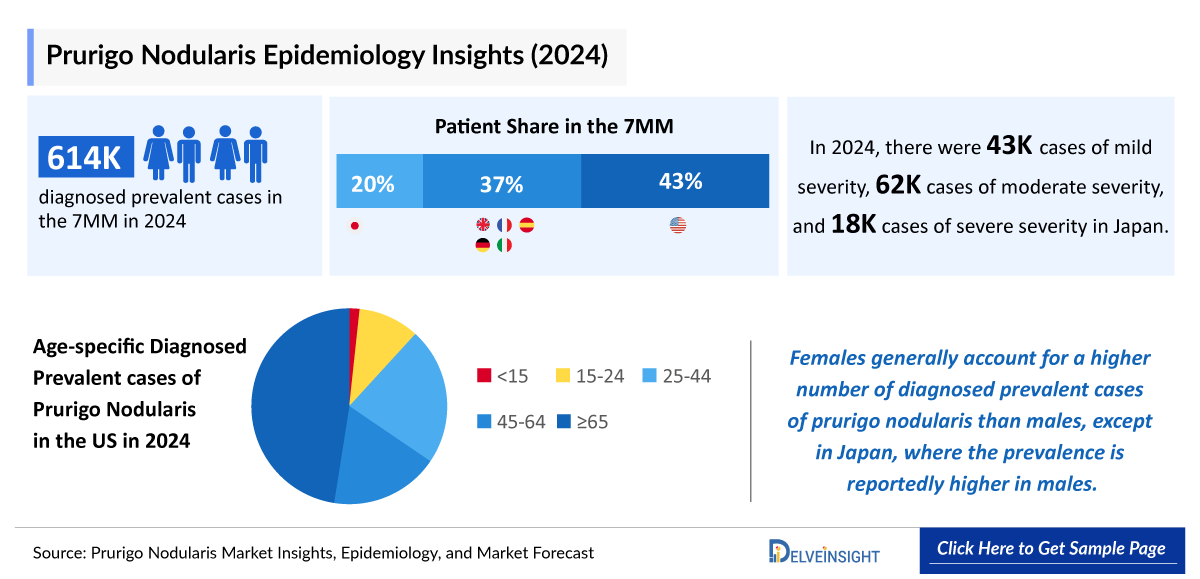
- The total diagnosed prevalent cases of prurigo nodularis in the 7MM were ~614,000 in 2024, out of which the highest prevalent cases of this disease were in the United States.
- Females generally account for a higher number of diagnosed prevalent cases of prurigo nodularis than males, with the exception of Japan, where the prevalence is reportedly higher in males.
- DUPIXENT (dupilumab) and NEMLUVIO (nemolizumab) are approved treatments for prurigo nodularis. DUPIXENT targets IL-4 and IL-13 cytokines, reducing inflammation and itching, while NEMLUVIO targets IL-31 to alleviate severe itching. Both drugs offer effective options for managing moderate-to-severe cases, improving symptoms and quality of life.
- Key Prurigo Nodularis players such as Amgen/Kyowa Kirin (rocatinlimab), Incyte (povorcitinib and ruxolitinib), Celldex (barzolvolimab), and others are evaluating their lead candidates in different stages of clinical development, respectively and will significantly impact the prurigo nodularis market during the forecast period (2025-2034).
- Emerging treatments with novel mechanisms of action may offer more effective management options for prurigo nodularis. Growing awareness and improved diagnostic techniques are crucial for early detection and better patient outcomes.
- According to the Q1 2025 presentation, Kyowa Kirin stated that Phase III completion for rocatinlimab in prurigo nodularis is expected in 2027.
DelveInsight’s "Prurigo Nodularis Market Insight, Epidemiology, and Market Forecast – 2034" report delivers an in-depth understanding of prurigo nodularis, historical and forecasted epidemiology as well as the prurigo nodularis therapeutics market trends in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
The prurigo nodularis market report provides current treatment practices, emerging drugs, prurigo nodularis market share of individual therapies, and current and forecasted prurigo nodularis market size from 2020 to 2034, segmented by seven major markets. The report also covers current prurigo nodularis treatment practices/algorithms and unmet medical needs to curate the best of the opportunities and assess the underlying potential of the market.
|
Study Period |
2020 to 2034 |
|
Forecast Period |
2025-2034 |
|
Geographies Covered |
|
|
Prurigo Nodularis Market |
|
|
Prurigo Nodulariss Market Size | |
|
Prurigo Nodularis Companies |
Sanofi, Regeneron, Galderma, Maruho, Amgen/Kyowa Kirin, Incyte Corporation, Keymed Biosciences, Celldex Therapeutics, and others. |
|
Prurigo Nodularis Epidemiology Segmentation |
|
Key Factors Driving the Prurigo Nodularis Market
Rising Prurigo Nodularis Disease Prevalence and Awareness
The increasing prevalence of prurigo nodularis, particularly among individuals with underlying skin conditions like atopic dermatitis and eczema, has heightened the demand for effective treatments. The US accounted for approximately 261K diagnosed prevalent cases of prurigo nodularis in 2024. These cases are expected to increase during the forecast period (2025−2034) owing to growing populations, improved diagnostic methods, changes in lifestyle and environmental factors, and advancements in medical technology, allowing for better treatment. Moreover, there is a growing awareness among healthcare professionals and patients about the condition, leading to earlier diagnosis and intervention.
Drug Approvals in Prurigo Nodularis Market
The approval of DUPIXENT (dupilumab) in 2022 and NEMLUVIO (nemolizumab) in 2024 provides a significant breakthrough for patients with moderate-to-severe prurigo nodularis. These prurigo nodularis therapies offer targeted therapies with proven efficacy. As more treatments are developed and approved, expanding access to biologic therapies like DUPIXENT and NEMLUVIO through improved insurance coverage and cost reduction programs could make these therapies more accessible to a broader range of patients.
Emerging Therapies in Prurigo Nodularis Market
The emerging prurigo nodularis clinical trial landscape offers a diverse range of therapeutic alternatives, including Povorcitinib (Incyte), Ruxolitinib (Incyte), Rocatinlimab (Amgen/Kyowa Kirin), Barzolvolimab (CDX-0159) (Celldex Therapeutics), Vixarelimab (Kiniksa Pharmaceuticals/Genentech), and others across various treatment lines. The expected launch of these therapies shall further create a positive impact on the prurigo nodularis market.
Prurigo Nodularis Treatment Market
Prurigo Nodularis Overview
Prurigo nodularis is a chronic skin disorder characterized by the appearance of multiple, firm, itchy nodules on the skin, which are often the result of repetitive scratching or rubbing. These nodules can range in size and are typically found on areas like the arms, legs, back, and chest. The primary symptom of prurigo nodularis is intense pruritus (itching), which causes individuals to scratch, further aggravating the lesions and leading to thickened, scaly, or hyperpigmented skin. In severe cases, the nodules can ulcerate or become infected due to ongoing friction and trauma.
The exact cause of prurigo nodularis remains unclear, but it is frequently associated with a variety of underlying conditions such as atopic dermatitis, chronic kidney disease, liver disease, and neuropsychiatric disorders like anxiety or depression. The condition often presents as a manifestation of immune system dysregulation, chronic inflammation, and nerve involvement. Prurigo nodularis can significantly impact an individual’s quality of life, as the persistent itching and skin lesions can interfere with daily activities, sleep, and emotional well-being.
Prurigo Nodularis Diagnosis
The diagnosis of prurigo nodularis is primarily clinical, based on the characteristic appearance of itchy, raised nodules on the skin. A healthcare provider will typically assess the patient's medical history, including any underlying conditions such as atopic dermatitis, kidney disease, or mental health issues that may contribute to the development of prurigo nodularis. A physical examination is conducted to evaluate the number, size, and location of the nodules, which are typically found on the arms, legs, and torso.
In some cases, a skin biopsy may be performed to rule out other conditions, such as skin infections or malignancies, as the nodules may resemble other dermatologic disorders. Laboratory tests may also be conducted to assess for underlying systemic conditions, particularly in patients with suspected renal or hepatic disease. The diagnosis is often confirmed by the persistence of lesions and a pattern of intense itching, which is key in distinguishing prurigo nodularis from other dermatological conditions.
Further details related to diagnosis will be provided in the report…
Prurigo Nodularis Treatment
The treatment of prurigo nodularis focuses on relieving the intense itching and inflammation, while addressing the underlying causes if identified. First-line treatments typically include topical therapies such as potent corticosteroids to reduce inflammation and suppress the immune response. Topical calcineurin inhibitors, like tacrolimus, may also be used as an alternative to steroids, particularly for sensitive areas. Emollients and moisturizing creams are recommended to maintain skin hydration and prevent further irritation. Ongoing Prurigo Nodularis clinical trials are exploring novel therapies to address this chronic skin condition, aiming to improve itch relief, lesion clearance, and overall quality of life for patients.
For more severe cases, systemic treatments may be considered. Oral antihistamines can help reduce itching, while oral corticosteroids may be prescribed for short-term flare-ups. Immunosuppressive drugs like methotrexate, cyclosporine, or azathioprine are sometimes used to control inflammation in refractory cases. Phototherapy, specifically narrowband ultraviolet B (NB-UVB) light therapy, is another effective treatment option that can help improve symptoms by modulating immune function and reducing skin inflammation.
In cases where prurigo nodularis is associated with an underlying condition, such as kidney disease or psychiatric disorders, treating the primary illness may help alleviate the skin symptoms. Psychological support, including cognitive-behavioral therapy or counseling, may also benefit patients experiencing significant stress or anxiety related to the condition. Due to the chronic nature of PN, a combination of therapies is often required to achieve symptom control and improve the patient’s quality of life.
Further details related to treatment will be provided in the report…
Prurigo Nodularis Epidemiology
The prurigo nodularis epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by the diagnosed prevalent cases of prurigo nodularis, gender-specific diagnosed prevalent cases of prurigo nodularis, age-specific diagnosed prevalent cases of prurigo nodularis, and severity-specific diagnosed prevalent cases of prurigo nodularis in the 7MM covering the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan from 2020 to 2034.
Key Findings from Prurigo Nodularis Epidemiological Analyses and Forecast
- The total diagnosed prevalent cases of prurigo nodularis in the US were around 261,900 cases in 2024.
- The US contributed to the largest diagnosed prevalent population of prurigo nodularis, acquiring ~43% of the 7MM in 2024. Whereas EU4 and the UK, and Japan accounted for around 37% and 20% of the total population share, respectively, in 2024.
- According to DelveInsight estimates, in 2024, among the age-specific diagnosed prevalent cases of prurigo nodularis in the US, the highest number of cases were found in the =65 years age group (~124,400), followed by 25–44 year age group while the lowest number of cases was observed in <15 years age group.
- The diagnosed prevalent cases of prurigo nodularis were distributed on the basis of severity; mild, moderate, and severe. In 2024, severe cases (~137,850) were most prevalent followed by moderate and mild cases in the US.
- In Japan, the diagnosed prevalent cases of prurigo nodularis were more in male; ~70,100 then in females in 2024.
- Among EU4, Germany accounted for the largest number of diagnosed prevalent cases followed by France, whereas Spain accounted for the lowest cases in 2024.
- Patients with prurigo nodularis face many challenges, including delayed diagnosis or misdiagnosis due to lack of awareness by patients and providers; unpredictable disease course.
Prurigo Nodularis Epidemiology Segmentation
- Diagnosed Prevalent Cases of Prurigo Nodularis
- Gender-specific Diagnosed Prevalent Cases of Prurigo Nodularis
- Age-specific Diagnosed Prevalent Cases of Prurigo Nodularis
- Severity-specific Diagnosed Prevalent Cases of Prurigo Nodularis
Prurigo Nodularis Market Recent Developments and Breakthroughs
- In March 2025, Incyte announced the results of Phase III clinical trials (TRuE-PN1 and TRuE-PN2 studies) evaluating ruxolitinib cream 1.5% (OPZELURA) in patients with prurigo nodularis at the 2025 American Academy of Dermatology (AAD) Annual Meeting.
- In February 2025, Galderma received marketing authorization for NEMLUVIO from both the UK’s Medicines and Healthcare Products Regulatory Agency (MHRA) and the European Commission (EC) for the SC treatment of adults with moderate-to-severe prurigo nodularis who are candidates for systemic therapy.
- In October 2024, Incyte’s Q3 2024 report included that the launch of povorcitinib for the treatment of prurigo nodularis is expected to be by 2027–2028.
- In October 2024, Incyte’s Q3 2024 report included that the launch of povorcitinib for the treatment of prurigo nodularis is expected to be by 2027–2028.
- In September 2024, Incyte presented long-term extension data at the 2024 EADV Congress from the Phase II randomized, double-blind, placebo-controlled study evaluating the efficacy and safety of povorcitinib in patients with prurigo nodularis
- In March 2024, Incyte presented new late-breaking data from its Phase II study evaluating povorcitinib (INCB54707), an oral JAK1 inhibitor, in adult patients with prurigo nodularis. The study met its primary and secondary endpoints following 16 weeks of treatment across all dosing groups. The results were shared as a late-breaking oral presentation at the AAD Annual Meeting, marking Incyte’s first presentation of data on prurigo Nodularis.
Prurigo Nodularis Drug Analysis
Drug chapter segment of the Prurigo Nodularis report encloses the detailed analysis of Prurigo Nodularis marketed drugs and late stage (Phase-III and Phase-II) Prurigo Nodularis pipeline drugs. It also helps to understand the Prurigo Nodularis clinical trial details, expressive pharmacological action, agreements and collaborations, approval and patent details, advantages and disadvantages of each included drug and the latest news and press releases. The Prurigo Nodularis drugs market is witnessing growth due to rising incidences of gastrointestinal disorders, increasing awareness, and ongoing clinical trials focused on innovative therapies and targeted treatment options. The Prurigo Nodularis Drugs market is expanding as novel biologics and small molecules target chronic itching and nodular lesions, driven by unmet clinical needs, regulatory support, and increasing epidemiological awareness.
Prurigo Nodularis Marketed Drugs
NEMLUVIO/MITCHGA (nemolizumab-ilto): Galderma Laboratories/Maruho
NEMLUVIO is the first and only treatment that blocks IL-31 signaling, a key driver of itch, inflammation, and the formation of prurigo nodularis nodules, by relieving itch and reducing the urge to scratch, and allowing skin to heal. NEMLUVIO is intended for adults who have been diagnosed with prurigo nodularis. It is the only self-injectable treatment for prurigo nodularis that is administrated approximately once a month.
Nemolizumab has received multiple international regulatory approvals for the treatment of moderate-to-severe prurigo nodularis, including US FDA approval in August 2024 for NEMLUVIO as a pre-filled pen for adults; Japan’s MHLW approval in March 2024 for MITCHGA SC injection for patients aged 13 and older; and marketing authorizations in February 2025 from both the UK MHRA and the European Commission for subcutaneous use in adults who are candidates for systemic therapy.
Comparison of Prurigo Nodularis Marketed Assets | ||||||
|
Drug |
Company |
Indication |
Molecule Type |
MoA |
RoA |
Marketed Region |
|
DUPIXENT (dupilumab) |
Sanofi/ |
Prurigo Nodularis |
Monoclonal antibody |
IL-13/4 receptor antagonists |
SC |
US: 2022; EU: 2022; JP: 2023 |
|
NEMLUVIO/ |
Galderma/Maruho |
Prurigo Nodularis |
Monoclonal antibody |
IL-31 receptor antagonists |
SC |
US: 2024; EU: 2025; JP: 2024 |
Prurigo Nodularis Emerging Drugs
Povorcitinib: Incyte
Povorcitinib is an oral small-molecule JAK1 inhibitor. Janus kinases (JAKs) are intracellular signaling enzymes that act downstream of key proinflammatory cytokines to mediate normal immune function and modulate inflammation. Currently, povorcitinib is in Phase III clinical trials for prurigo nodularis, hidradenitis suppurativa, and vitiligo. In April 2024, Incyte and China Medical System Holdings announced that the companies entered into a collaboration and license agreement through a wholly-owned dermatology medical aesthetic subsidiary, CMS Skinhealth, for the development and commercialization of povorcitinib, a selective oral JAK1 inhibitor, in Mainland China, Hong Kong, Macau, Taiwan Region and eleven Southeast Asian countries.
The company anticipates the data release of the povorcitinib Phase III trial for prurigo nodularis by 2026.
Barzolvolimab (CDX-0159): Celldex Therapeutics
Barzolvolimab is a humanized monoclonal antibody that specifically binds the receptor tyrosine kinase KIT with high specificity and potently inhibits its activity. KIT is expressed in a variety of cells, including mast cells, which mediate inflammatory responses such as hypersensitivity and allergic reactions. KIT signaling controls the differentiation, tissue recruitment, survival, and activity of mast cells. In certain inflammatory diseases, mast cell activation plays a central role in the onset and progression of the disease. The company initiated a Phase II study in prurigo nodularis, and enrollment is ongoing; positive data from a Phase I study in prurigo nodularis was reported in November 2023.
In May 2024, Celldex Therapeutics announced the first patient was administrated with dosed in the Company’s Phase II subcutaneous study of barzolvolimab in prurigo nodularis.
Comparison of Prurigo Nodularis Emerging Drugs Under Development | ||||||
|
Product |
Company |
Indication |
Phase |
Molecule type |
MOA |
ROA |
|
Rocatinlimab |
Amgen/Kyowa Kirin |
Prurigo Nodularis |
III |
Monoclonal antibody |
OX40 ligand inhibitors |
SC |
|
Povorcitinib |
Incyte |
Prurigo Nodularis |
III |
Small molecule |
JAK 1 inhibitors |
Oral |
|
OPZELURA (ruxolitinib) |
Incyte |
Prurigo Nodularis |
III |
Small molecule |
JAK 1/2inhibitors |
Topical |
|
Stapokibarta |
Keymed Biosciences |
Prurigo Nodularis |
III |
Monoclonal antibody |
IL 4 receptor alpha subunit antagonists |
SC |
|
Nalbuphineb |
Trevi Therapeutics |
Prurigo Nodularis |
II/III |
Small Molecule |
Opioid mu receptor antagonists |
Oral |
|
Barzolvolimab |
Celldex Therapeutics |
Prurigo Nodularis |
II |
Monoclonal antibody |
KIT antagonist |
SC |
|
Vixarelimabd |
Kiniksa Pharmaceuticals /Genentech |
Prurigo Nodularis |
II |
Monoclonal antibody |
OSMRβ inhibitor |
SC |
|
Abrocitinibc |
Pfizer |
Prurigo Nodularis |
II |
Small Molecule |
JAK 1/2 inhibitors |
Oral |
|
a. The study trial going on in China but not conducted in 7MM. b. The study discontinuation due to treatment-related adverse events occurred in 13% of patients. c. University trials funded by Pfizer and drug was not present in the pipeline of the company. d. In a press release from December 2023, the PCORI noted that Kiniksa had canceled its plan to release data from the Phase IIb clinical trial of vixarelimab in prurigo nodularis, which was initially expected in the second half of 2022. Additionally, Genentech is no longer pursuing the development of vixarelimab for this indication. | ||||||
Prurigo Nodularis Drug Class Analysis
Corticosteroids
Topical corticosteroids are the first-line treatment for mild to moderate prurigo nodularis, working by reducing inflammation, suppressing immune responses, and constricting blood vessels to alleviate itching and inflammation. Potent corticosteroids, such as betamethasone dipropionate, clobetasol propionate, and fluocinonide, are effective for areas with thicker skin or resistant lesions. They provide rapid relief but are used for short durations to avoid side effects like skin thinning, delayed wound healing, and stretch marks.
Systemic corticosteroids, like oral prednisone, are used for severe or widespread prurigo nodularis that doesn't respond to other treatments. They control inflammation and generalized itching but are typically prescribed for short durations (weeks to months) to avoid side effects such as weight gain, osteoporosis, and increased infection risk. The goal is to provide temporary relief while adjusting other treatments.
Antihistamines and leukotriene inhibitors
Antihistamines are commonly used in prurigo nodularis treatment due to the high presence of mast cells in prurigo nodularis lesions. A case series showed that high-dose nonsedating antihistamines during the day and sedating antihistamines at night improved symptoms in chronic prurigo patients. A combination therapy of fexofenadine and montelukast also improves lesions and pruritus in some prurigo nodularis patients.
Janus kinase (JAK) inhibitors
Janus kinase (JAK) inhibitors are emerging as a promising treatment for prurigo nodularis, particularly for patients with moderate to severe disease. JAK inhibitors work by targeting specific enzymes involved in the inflammatory process, which can help reduce itching and the formation of skin lesions in prurigo nodularis. By inhibiting the JAK-STAT signaling pathway, these drugs block the activity of pro-inflammatory cytokines such as interleukins (IL-4, IL-13, IL-31) and interferons, which play a key role in the pathogenesis of prurigo nodularis. This action can reduce both inflammation and pruritus, providing relief from the symptoms of prurigo nodularis.
Further detailed analysis will be provided in the report….
Prurigo Nodularis Market Outlook
Prurigo nodularis is a chronic skin condition marked by hard, itchy bumps (nodules) caused by persistent scratching and rubbing. The nodules typically appear on areas like the arms, legs, abdomen, and back, but rarely on the palms, soles, or face. The cycle of itching and scratching worsens the condition, leading to new nodules and potential scarring. Treatment focuses on breaking this itch-scratch cycle to promote healing.
The treatment landscape for prurigo nodularis has been evolving in recent years, with increasing attention from the pharmaceutical industry due to the chronic and debilitating nature of the condition. There is growing recognition of the need for effective treatments that can break the persistent itch-scratch cycle that exacerbates the disease.
Treatments for prurigo nodularis focus on relieving itching and inflammation. Topical corticosteroids, including strong steroid creams, are commonly prescribed to reduce skin inflammation and itching. Tacrolimus ointment, a non-steroid option, also helps reduce inflammation. Applying paste bandages or cling film over the affected areas enhances the effect of steroids and prevents excessive scratching. Emollients are crucial for moisturizing dry skin and improving the effectiveness of other treatments. Antihistamines, particularly non-sedative types like fexofenadine, help relieve itching. Phototherapy, such as UVB or UVA treatments, reduces nodules and itching. Psychological support may aid in managing stress and emotional distress, while immune-suppressing treatments like oral corticosteroids, ciclosporin, methotrexate, or azathioprine are used for severe cases.
- The Prurigo Nodularis market in the 7MM was valued at approximately USD 1,488 million in 2025 and is projected to reach USD 4,433 million by 2034 at a CAGR of 12.90% over the forecast period from 2024 to 2034.
- Among the 7MM, the US accounted for the largest market size of prurigo nodularis. i.e., USD ~810 million in 2024.
- Among EU4 and the UK, Germany accounted for the highest market size in 2024, while Spain occupied the lowest.
- In 2024, among all the current therapies for prurigo nodularis, the highest revenue was generated by DUPIXENT, i.e., nearly USD 630 million in the United States.
Further details will be provided in the report….
Prurigo Nodularis Drugs Uptake
This section focuses on the uptake rate of potential drugs expected to be launched in the market during 2025–2034. The landscape of prurigo nodularis treatment has experienced an uptake of novel drugs. These innovative therapies are redefining standards of care. Furthermore, the increased uptake of these transformative drugs is a testament to the unwavering dedication of physicians, oncology professionals, and the entire healthcare community in their tireless pursuit of advancing treatment care. This momentous shift in treatment paradigms is a testament to the power of research, collaboration, and human resilience.
Although there are very few approved medications for prurigo nodularis, several drugs are currently under research and development. These include rocatinlimab (Amgen/Kyowa Kirin), povorcitinib and ruxolitinib (Incyte), and barzolvolimab (Celldex), which are being investigated as potential treatments for prurigo nodularis.
The discontinuation of treatment due to adverse events in 13% of nalbuphine-treated patients highlights safety concerns that may impact its clinical utility. Meanwhile, the halted development and withheld trial data for vixarelimab by both Kiniksa and Genentech reflect significant setbacks in advancing therapeutic options for prurigo nodularis, underscoring the ongoing challenges in finding effective and tolerable treatments for this condition.
Further detailed analysis of emerging therapies drug uptake in the report…
Prurigo Nodularis Pipeline Development Activities
The Prurigo Nodularis pipeline report provides insights into different Prurigo Nodularis clinical trials within Phase III, Phase II, and Phase I stage. It also analyzes key players involved in developing targeted therapeutics.
Prurigo Nodularis Pipeline Development Activities
The Prurigo Nodularis clinical trials analysis report covers detailed information on collaborations, acquisitions and mergers, licensing, and patent details for prurigo nodularis emerging therapies.
Latest KOL Views on Prurigo Nodularis
To keep up with current market trends, we take KOLs and SMEs' opinions working in the domain through primary research to fill the data gaps and validate our secondary research. Some of the leaders like MD, Professor and Vice Chair Department of Critical Care Medicine and Director, PhD, and others. Their opinion helps to understand and validate current and emerging therapies and treatment patterns or prurigo nodularis market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
What KOLs are saying on Prurigo Nodularis Patient Trends?
Delveinsight’s analysts connected with 20+ KOLs to gather insights; however, interviews were conducted with 10+ KOLs in the 7MM. Centers such as University of Miami, US; University of Lübeck, Germany; Università Cattolica del Sacro Cuore, Italy; Johns Hopkins University School of Medicine, US; University Hospital Münster, Münster, Germany; Johns Hopkins University School of Medicine, US; Massachusetts General Hospital, US; Nagasaki University, Japan; Department of Dermatology, Nagasaki University School of Medical Sciences, Nagasaki, Japan, etc., were contacted. Their opinion helps understand and validate prurigo nodularis epidemiology and market trends.
|
KOL Views |
|
“Current treatments for prurigo nodularis are limited, there is hope on the horizon. Research into new therapies targeting specific pathways involved in pruritus and inflammation is ongoing. Agents that target neuroimmune interactions or modulate the itch-scratch cycle specifically could offer patients much-needed relief. As the understanding of PN’s pathophysiology continues to evolve, it’s likely that more effective, personalized treatment options will become available.” -Researcher, Nagasaki University School of Medical Sciences, Japan |
|
“Recent advances in biologic therapies, particularly DUPIXENT, have shown potential in treating refractory prurigo nodularis, especially when it’s associated with atopic dermatitis. These therapies target specific immune pathways, offering a promising alternative to systemic corticosteroids and immunosuppressants. However, access to biologics remains a challenge due to cost and limited availability in some regions.” -PhD, Massachusetts General Hospital, US |
Prurigo Nodularis Report Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT and conjoint analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the Analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving treatment landscape.
The analyst analyzes multiple emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry.
In efficacy, the trial’s primary and secondary outcome measures are evaluated.
Further, the therapies’ safety is evaluated wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials.
Prurigo Nodularis Market Access and Reimbursement
DUPIXENT: MyWay CoPay program
The eligible patients covered by commercial health insurance may pay as little as a USD 0 co-pay per fill of DUPIXENT (maximum of USD 13,000 per patient per calendar year).
Key eligibility criteria:
- Patients are eligible for the program if they meet all of the following criteria:
- The patient currently has commercial (private) health insurance that covers DUPIXENT.
- The patient has a DUPIXENT prescription for an FDA-approved condition.
- Patients are residents of the 50 United States, the District of Columbia, Puerto Rico, Guam, or the USVI and are patients or caregivers aged 18 years or older.
Further detailed analysis will be provided in the report…
Scope of the Prurigo Nodularis Market Report
- The report covers a descriptive overview of prurigo nodularis, explaining its causes, signs and symptoms, pathogenesis, and currently available therapies.
- Comprehensive insight has been provided into prurigo nodularis epidemiology and treatment.
- Additionally, an all-inclusive account of both the current and emerging therapies for prurigo nodularis is provided, along with the assessment of new therapies, which will have an impact on the current treatment landscape.
- A detailed review of the prurigo nodularis market; historical and forecasted is included in the report, covering the 7MM drug outreach.
- The report provides an edge while developing business strategies, by understanding trends shaping and driving the 7MM prurigo nodularis market.
Prurigo Nodularis Market Report Insights
- Prurigo Nodularis Patient Population
- Prurigo Nodularis Therapeutic Approaches
- Prurigo Nodularis Pipeline Analysis
- Prurigo Nodularis Market Size and Trends
- Prurigo Nodularis Market Opportunities
- Impact of Upcoming Prurigo Nodularis Therapies
Prurigo Nodularis Market Report Key Strengths
- Ten Years Forecast
- 7MM Coverage
- Prurigo Nodularis Epidemiology Segmentation
- Key Cross Competition
- Highly Analyzed Market
- Prurigo Nodularis Drugs Uptake
Prurigo Nodularis Market Report Assessment
- Current Prurigo Nodularis Treatment Practices
- Prurigo Nodularis Unmet Needs
- Prurigo Nodularis Pipeline Product Profiles
- Prurigo Nodularis Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
- Prurigo Nodularis Market Drivers
- Prurigo Nodularis Markrt Barriers
FAQs
- What was the prurigo nodularis market share (%) distribution in 2020 and what it would look like in 2034?
- What would be the prurigo nodularis total market size as well as market size by therapies across the 7MM during the study period (2020–2034)?
- Which country will have the largest prurigo nodularis market size during the study period (2020–2034)?
- What are the disease risks, burdens, and unmet needs of prurigo nodularis?
- What is the historical prurigo nodularis patient pool in the United States, EU4 (Germany, France, Italy, and Spain), and the UK, and Japan?
- What will be the growth opportunities across the 7MM concerning the patient population of prurigo nodularis?
- How many emerging therapies are in the mid-stage and late stage of development for the treatment of prurigo nodularis?
- What are the key collaborations (Industry–Industry, Industry-Academia), Mergers and acquisitions, and licensing activities related to prurigo nodularis therapies?
- What are the recent novel therapies, targets, mechanisms of action, and technologies developed to overcome the limitations of existing therapies?
- What are the clinical studies going on for prurigo nodularis and their status?
- What are the key designations that have been granted for the emerging therapies for prurigo nodularis?
Reasons to buy Prurigo Nodularis Market Forecast Report
- The report will help in developing business strategies by understanding trends shaping and driving prurigo nodularis.
- To understand the future market competition in the prurigo nodularis market and Insightful review of the SWOT analysis of prurigo nodularis.
- Organize sales and marketing efforts by identifying the best opportunities for prurigo nodularis in the US, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
- Identification of strong upcoming players in the market will help in devising strategies that will help in getting ahead of competitors.
- Organize sales and marketing efforts by identifying the best opportunities for the prurigo nodularis market.
- To understand the future market competition in the prurigo nodularis market.