Pemphigus Vulgaris Market
- In the total Pemphigus Vulgaris market size in the 7MM, the United States accounted for the highest Pemphigus Vulgaris market share, i.e. more than 75% in 2023, followed by Germany.
- According to the National Organization for Rare Disorders (2023), pemphigus vulgaris (PV) is a severe, rare autoimmune disorder marked by painful blisters and erosions on the skin and mucous membranes, including the mouth, throat, and sometimes genital areas. It occurs when the immune system mistakenly attacks desmogleins, proteins essential for cell adhesion in the epidermis, leading to cell separation and blister formation.
- Blisters associated with pemphigus are typically flaccid and fragile, forming mainly on areas like the scalp, face, chest, and back, and particularly within mucous membranes such as the mouth. They rupture easily, often resulting in painful, open sores that are slow to heal. Unlike pemphigoid, which affects deeper skin layers and produces firmer, more resilient blisters, pemphigus involves more superficial skin layers, resulting in delicate, easily ruptured blisters.
- In Pemphigus Vulgaris, autoantibodies specifically target desmogleins (proteins crucial for cell-to-cell adhesion) in the epidermis. This leads to the breakdown of cellular connections, resulting in the separation of skin cells (acantholysis) and subsequent blister formation.
- Diagnosing Pemphigus Vulgaris involves differentiating it from other blistering diseases, such as bullous pemphigoid or other types of pemphigus, through biopsy and antibody testing, particularly identifying anti-desmoglein antibodies.
- Treatment focuses on controlling the autoimmune activity that causes blistering, primarily through corticosteroids, immunosuppressants like azathioprine or mycophenolate, and biologic therapy with Rituximab, which targets B-cells responsible for autoantibody production. Currently, RITUXAN (rituximab) is the only FDA-approved therapy for the treatment of pemphigus vulgaris; it has become an effective option for achieving remission, especially in moderate-to-severe cases, often allowing for lower corticosteroid doses.
- Additional supportive treatments include antibiotics for infection prevention, pain management for oral lesions, and proper wound care to aid healing. Despite a tendency for relapse, close monitoring and these therapies help many patients manage PV, and new-targeted treatments are under investigation to improve outcomes further.
- CABA-201, a CD19-CAR T therapy developed by Cabaletta Bio, is in Phase I/II development for treating PV.
- In 2023, the United States accounted for the highest diagnosed prevalent cases of Pemphigus Vulgaris followed by Germany.
- In the 7MM, Females reported more cases than males for Pemphigus Vulgaris.
- In the United States, individuals of the 60-69 year age group reported the highest number of pemphigus Vulgaris cases.
Pemphigus Vulgaris Market Report Summary
- The Pemphigus Vulgaris market report offers extensive knowledge regarding the epidemiology segments and predictions, presenting a deep understanding of the potential future growth in diagnosis rates, disease progression, and treatment guidelines. It provides comprehensive insights into these aspects, enabling a thorough assessment of the subject matter.
- Additionally, an all-inclusive account of the current management techniques and emerging therapies and the elaborative profiles of late-stage (Phase III and Phase II) and prominent therapies that would impact the current treatment landscape and result in an overall market shift has been provided in the report.
- The Pemphigus Vulgaris market report also encompasses a comprehensive analysis of the Pemphigus Vulgaris market, providing an in-depth examination of its historical and projected Pemphigus Vulgaris market size (2020–2034). It also includes the Pemphigus Vulgaris market share of therapies, detailed assumptions, and the underlying rationale for our methodology. The report also includes drug outreach coverage in the 7MM region.
- The Pemphigus Vulgaris market report includes qualitative insights that provide an edge while developing business strategies by understanding trends through SWOT analysis and expert insights/KOL views, including experts from various hospitals and prominent universities, patient journey, and treatment preferences that help shape and drive the 7MM Pemphigus Vulgaris market.
Key Factors Driving Pemphigus Vulgaris Market
Rising Pemphigus Vulgaris Prevalence in Aging Populations
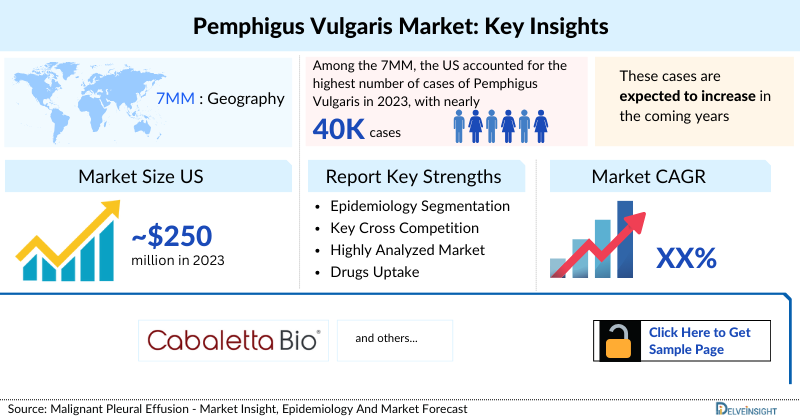
In the 7MM, Pemphigus Vulgaris cases continue to rise, with the United States accounting for nearly 40K cases in 2023, over 75% of the total market share. Prevalence is notably higher among females, and the highest number of cases is reported in individuals aged 60–69 years. By 2034, diagnosed cases are projected to increase further, reflecting both demographic shifts and improved diagnostic awareness.
Launch of Emerging Biologic and Cell-Based PV Therapies
RITUXAN (rituximab) remains the only FDA-approved therapy for Pemphigus Vulgaris, serving as a cornerstone in moderate-to-severe cases. However, the pipeline is advancing with innovative therapies, most notably Cabaletta Bio’s CABA-201, a CD19-CAR T-cell therapy currently in Phase I/II clinical development. Designed to transiently deplete CD19-positive B cells, CABA-201 aims to “reset” the immune system and deliver long-term remission with a single infusion. Additional approaches under investigation include novel B-cell–directed antibodies and antigen-specific cell therapies.
Expanding R&D and Clinical Trial Investments in PV
Over a dozen pharmaceutical and biotech companies, including Cabaletta Bio, Roche, Sanofi, Novartis, and others, are investing in next-generation Pemphigus Vulgaris therapies. Multiple RESET Phase I/II trials are ongoing for CABA-201 across autoimmune indications, with pemphigus cohorts advancing in parallel. The orphan-drug designation previously granted to DSG3-CAART underscores the increasing regulatory and commercial interest in targeted PV therapies.
Advances in Diagnostics and Early Disease Management
Improved diagnostic accuracy, through antibody testing for anti-desmoglein 1 and 3 and confirmatory skin biopsies, has enabled more precise disease differentiation from related blistering disorders, such as bullous pemphigoid. Early detection now accounts for a growing share of identified cases, allowing timely intervention with corticosteroids, immunosuppressants, and biologics. This shift toward earlier diagnosis and personalized therapy has already begun to improve outcomes and reduce relapse frequency.
Pemphigus Vulgaris Market
- A few key Pemphigus Vulgaris companies are leading the treatment landscape, such as Roche, Cabaletta Bio, and others. The details of the country-wise and therapy-wise Pemphigus Vulgaris market size have been provided below.
- In the total Pemphigus Vulgaris market size in the 7MM, the United States accounted for the highest Pemphigus Vulgaris market share, i.e. more than 75% in 2023, followed by Germany.
- Among EU4 and the UK, Germany accounted for almost 25% of the Pemphigus Vulgaris market size in 2023.
- The United States Pemphigus Vulgaris Market accounted for approximately USD 250 million in 2023.
- Among the therapies available, Rituximab appears to be the drug that has transformed the Pemphigus Vulgaris market.
Pemphigus Vulgaris Drug Chapters
The section dedicated to drugs in the Pemphigus Vulgaris market report provides an in-depth evaluation of late-stage pipeline drugs (Phase III and Phase II) related to Pemphigus Vulgaris. The Pemphigus Vulgaris drug chapters section provides valuable information on various aspects related to clinical trials of Pemphigus Vulgaris, such as the pharmacological mechanisms of the drugs involved, designations, approval status, patent information, and a comprehensive analysis of the pros and cons associated with each drug. Furthermore, it presents the most recent news updates and press releases on drugs targeting Pemphigus Vulgaris.
Marketed Pemphigus Vulgaris Therapies
RITUXAN (rituximab): Roche
RITUXAN (rituximab) is the only FDA-approved therapy for the treatment of pemphigus vulgaris. It serves as a key treatment, especially for moderate to severe cases. As the first biologic therapy approved by the FDA for this condition, RITUXAN represents a significant advancement in over 60 years. The FDA has granted RITUXAN several important designations, including Priority Review, Breakthrough Therapy Designation, and Orphan Drug Designation, emphasizing its critical role in treating Pemphigus Vulgaris. Rituximab is a CD20-directed cytolytic antibody that targets B-cells, which are responsible for producing the autoantibodies that attack the skin and mucous membranes in Pemphigus Vulgaris. By depleting these B-cells, RITUXAN reduces the production of harmful antibodies, leading to improved symptoms and better disease control.
Emerging Pemphigus Vulgaris Therapies
CABA-201 (4-1BB CD19-CAR T): Cabaletta Bio
CABA-201 is designed to deeply and transiently deplete CD19-positive B cells following a one-time infusion, which may enable an “immune system reset” with the potential for durable remission of therapy in patients with autoimmune diseases. To date, Cabaletta has received clearance from the FDA for Investigational New Drug (IND) applications for CABA-201 in multiple autoimmune conditions, including Systemic Lupus Erythematosus (SLE), myositis, systemic sclerosis and generalized Myasthenia Gravis (gMG). Cabaletta is conducting four RESET Phase I/II clinical trials with a total of nine cohorts that can advance simultaneously, employing a similar parallel cohort design and starting dose of 1 x 106 cells/kg without a dose escalation requirement. In January 2020, Cabaletta Bio announced that the US Food and Drug Administration (FDA) had granted an Orphan Drug Designation for the Company’s lead product candidate, DSG3-CAART, for the treatment of pemphigus vulgaris.
Note: Detailed assessment will be provided in the final report of PV…
Pemphigus Vulgaris Market Outlook
Currently, RITUXAN (rituximab) is the only FDA-approved therapy for the treatment of pemphigus vulgaris. It serves as a key treatment, especially for moderate to severe cases. As the first biologic therapy approved by the FDA for this condition, RITUXAN represents a significant advancement in over 60 years.
A CD19-CAR T-cell therapy is being developed by Cabaletta Bio for treating autoimmune diseases, including Pemphigus Vulgaris. Currently, in mid-stage development, CABA-201 is designed to deeply and transiently deplete CD19-positive B cells following a one-time infusion, potentially enabling an "immune system reset" and durable disease remission. Cabaletta has received FDA clearance for Investigational New Drug (IND) applications for CABA-201 in several autoimmune conditions, and the therapy is being tested in multiple RESET Phase I/II clinical trials.
In a nutshell, not many potential Pemphigus Vulgaris therapies are being investigated to manage Pemphigus Vulgaris. Even though it is too soon to comment on the above-mentioned promising candidate to enter the Pemphigus Vulgaris market during the forecast period (2024–2034). Eventually, this drug will create a significant difference in the landscape of Pemphigus Vulgaris in the coming years. The treatment space is expected to experience a significant positive shift in the coming years owing to the improvement in healthcare spending worldwide.
Further details are provided in the report…
Pemphigus Vulgaris Treatment Market
Pemphigus Vulgaris Overview
Pemphigus is a rare autoimmune blistering disorder that affects the outermost layer of the skin (epidermis) and mucous membranes. It is characterized by the formation of fragile blisters and lesions that rupture easily, leaving painful sores. This condition arises when the immune system mistakenly produces autoantibodies—particularly against proteins called desmoglein 1 and desmoglein 3, which are essential for maintaining the adhesion between skin cells. The disruption of these cellular junctions leads to a loss of skin integrity, causing the skin cells to separate, a process known as acantholysis. As a result, fluid accumulates between the skin layers, leading to the formation of blisters.
Further details are provided in the report…
Pemphigus Vulgaris (PV) Diagnosis
The current diagnosis of Pemphigus Vulgaris involves a combination of clinical assessment, histopathological examination, and advanced immunological techniques such as direct and indirect immunofluorescence and ELISA. The diagnosis of Pemphigus Vulgaris should be suspected in any patient with mucocutaneous erosions or blisters. The oral mucosa is the first site of involvement in the majority of cases, and Pemphigus Vulgaris may remain confined to the mucosal surfaces or extend to involve the skin (average lag period of 4 months).
Further details related to country-based variations are provided in the report…
Pemphigus Vulgaris Treatment
The treatment of Pemphigus Vulgaris has significantly improved with the use of systemic corticosteroids, which reduced the disease's mortality rate by 60%. These steroids remain the first-line therapy, particularly for mild cases, though they require careful monitoring due to side effects like infections and osteoporosis. For moderate-to-severe PV, rituximab, an anti-CD20 monoclonal antibody, is commonly used in combination with corticosteroids, helping to deplete B-cells responsible for producing the autoantibodies that attack the skin. Steroid-sparing agents like azathioprine and mycophenolate mofetil (MMF) are also used to reduce steroid dependence and minimize side effects. For refractory cases, treatments such as IVIg and cyclophosphamide may be considered. While these therapies have improved outcomes, Pemphigus Vulgaris treatment remains complex, requiring ongoing management to balance efficacy and side effects.
Further details related to treatment and management are provided in the report…
Pemphigus Vulgaris Epidemiology
The Pemphigus Vulgaris epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by total diagnosed prevalent cases, Gender-specific cases, Age-specific cases, Severity-specific cases, and total treated cases of Pemphigus Vulgaris in the United States, EU4 countries (Germany, France, Italy, Spain) and the United Kingdom, and Japan from 2020 to 2034.
- Among the 7MM, the United States accounted for the highest number of cases of Pemphigus Vulgaris in 2023, with nearly 40,000 cases. These cases are anticipated to increase by 2034.
- In the United States, individuals of the 60-69 year age group account for the highest number of cases of Pemphigus Vulgaris in 2023.
- In the 7MM, the prevalence of females is more than males in Pemphigus Vulgaris.
- Among EU4 and the UK, Germany accounted for the highest number of diagnosed prevalent cases in 2023, while Spain accounted for the least.
KOL Views
To stay abreast of the latest trends in the Pemphigus Vulgaris market, we conduct primary research by seeking the opinions of Key Opinion Leaders (KOLs) and Subject Matter Experts (SMEs) who work in the relevant field. This helps us fill any gaps in data and validate our secondary research.
We have reached out to industry experts to gather insights on various aspects of Pemphigus Vulgaris, including the evolving treatment landscape, patients’ reliance on conventional therapies, their acceptance of therapy switching, drug uptake, and challenges related to accessibility. The experts we contacted included medical/scientific writers, professors, and researchers from prestigious universities in the US, Europe, the UK, and Japan.
Our team of analysts at Delveinsight connected with more than 15 KOLs across the 7MM. We contacted institutions such as the Johns Hopkins University School of Medicine, Stanford University School of Medicine, University of California, etc., among others. By obtaining the opinions of these experts, we gained a better understanding of the current and emerging treatment patterns in the Pemphigus Vulgaris market, which will assist our clients in analyzing the overall epidemiology and market scenario.
We perform Qualitative and Market Intelligence analysis using various approaches, such as SWOT analysis and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the Analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving treatment landscape.
Conjoint Analysis analyzes multiple approved and emerging Pemphigus Vulgaris therapies based on relevant attributes such as safety, efficacy, frequency of administration, designation, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy.
In efficacy, the trial’s primary and secondary outcome measures are evaluated; for instance, in trials for Pemphigus Vulgaris, one of the most important primary endpoints was achieving hemolysis control, LDH normalization, etc. Based on these, the overall efficacy is evaluated.
Further, the therapies’ safety is evaluated wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the route of administration, order of entry and designation, probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging Pemphigus Vulgaris therapies are decided.
Pemphigus Vulgaris Market Access and Reimbursement
Because newly authorized drugs are often expensive, some patients escape receiving proper treatment or use off-label, less expensive prescriptions. Reimbursement plays a critical role in how innovative treatments can enter the market. The cost of the medicine, compared to the benefit it provides to patients who are being treated, sometimes determines whether or not it will be reimbursed. Regulatory status, target population size, the setting of treatment, unmet needs, the number of incremental benefit claims, and prices can all affect market access and reimbursement possibilities.
The Pemphigus Vulgaris market report further provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of approved therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Pemphigus Vulgaris Market Report Insights
- Pemphigus Vulgaris Patient Population
- Pemphigus Vulgaris Therapeutic Approaches
- Pemphigus Vulgaris Market Size
- Pemphigus Vulgaris Market Trends
- Existing Pemphigus Vulgaris Market Opportunity
Pemphigus Vulgaris Market Report Key Strengths
- Eleven-year Forecast
- The 7MM Coverage
- Pemphigus Vulgaris Epidemiology Segmentation
- Key Cross Competition
Pemphigus Vulgaris Market Report Assessment
- Current Pemphigus Vulgaris Treatment Practices
- Reimbursements
- Pemphigus Vulgaris Market Attractiveness
- Qualitative Analysis (SWOT, Conjoint Analysis, Unmet needs)
Key Questions
- Would there be any changes observed in the current treatment approach?
- Will there be any improvements in Pemphigus Vulgaris management recommendations?
- Would research and development advances pave the way for future tests and therapies for Pemphigus Vulgaris?
- Would the diagnostic testing space experience a significant impact and lead to a positive shift in the treatment landscape of Pemphigus Vulgaris?
- What kind of uptake will the new therapies witness in the coming years in Pemphigus Vulgaris patients?
Related Insights: