Sjogren's Syndrome Market
- In 2023, the total Sjogren’s syndrome market size was around ~USD 1,900 million, which is expected to increase by 2034 during the study period (2020–2034) in the 7MM.
- Sjogren’s syndrome is an autoimmune disorder caused by the lymphocytic infiltration of exocrine glands resulting in glandular dysfunction, preferentially of the salivary and lacrimal glands.
- It can be classified into two types, i.e., primary Sjogren’s syndrome and secondary Sjogren’s syndrome. Primary Sjogren’s syndrome (pSS) occurs in the absence of other autoimmune diseases and is characterized by keratoconjunctiva sicca (dry eyes) and xerostomia (dry mouth), collectively called the sicca syndrome. In contrast, secondary Sjogren’s syndrome presents other autoimmune diseases such as rheumatoid arthritis and systemic lupus erythematosus (SLE).
- Dry eyes and dry mouth are the most common symptoms of SS. In Sjogren’s syndrome, the immune system also targets other body parts such as joints, thyroids, kidneys, liver, lungs, skin, and nerves. This results in joint pain, swelling, skin rashes, vaginal dryness, and others. It also affects other organ systems, which include skin, joints, muscles, blood, lungs, heart, kidneys, and nerves.
- The diagnosis of Sjogren’s syndrome is made based on medical history, physical examination, specific ocular, oral evaluation, blood tests, and salivary gland assessment (biopsy and ultrasound).
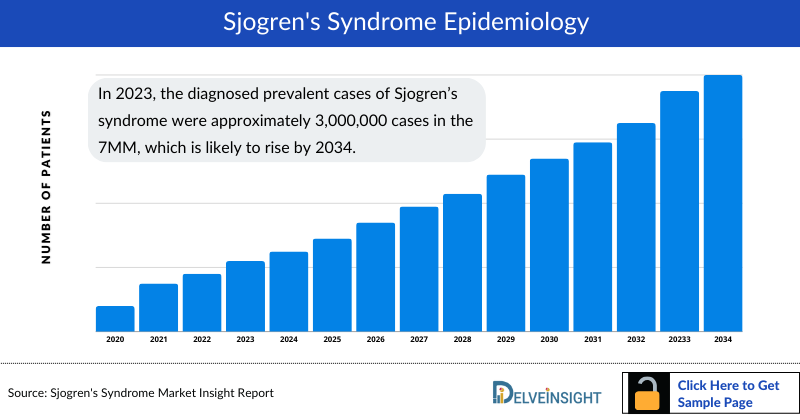
- In 2023, the diagnosed prevalent cases of Sjogren’s syndrome were approximately 3,000,000 cases in the 7MM, which is likely to rise by 2034.
- At present, there is no curing method for Sjogren’s syndrome. Treatment focuses on relieving symptoms using artificial tears and saliva, sugar-free candy, and secretagogue drugs such as pilocarpine or cevimeline. General symptoms such as pain and fatigue are frequently treated with hydroxychloroquine. Specific extraglandular involvement may require immunosuppressive drugs.
- Dry eyes usually respond to artificial tears applied regularly during the day or to gels applied at night. If patients develop yeast infections, anti-fungal therapies may be used. Humidifiers and nasal saline irrigation may improve nasal dryness. Medications that reduce gastric acid (such as proton pump inhibitors and H2 blockers) may lessen symptoms of acid reflux. Treatments may help relieve some of the dryness, but usually, some dryness persists.
- The first approach to extra-glandular (systemic) major organ-system disease is oral/parenteral corticosteroids. Various DMARDs (methotrexate, azathioprine) have been successfully employed as steroid-sparing agents, although none is specifically approved for Sjogren. Hydroxychloroquine is recommended to treat inflammatory polyarthritis; B cell depletion may have a role in certain severe extra-glandular manifestations (vasculitis). TNF-alpha inhibitors have not proved effective for Sjogren’s syndrome.
- The potential drugs that can mark a significant change in the forecast period include lanalumab (VAY736), CFZ533 (iscalimab), RSLV-132, Dazodalibep (VIB4920), OXERVATE (cenegermin), SOTYKTU (deucravacitinib), and others.
- In the total Sjogren’s syndrome market size in the 7MM, the United States accounted for the highest Sjogren’s syndrome market size, i.e., around 62% of the market in 2023.
- Among all the emerging therapies, CFZ533 (iscalimab) and VIB4920 (dazodalibep) are likely to have a major influence on the Sjogren’s syndrome market size in the 7MM.
- Several unmet needs thus still exist for the disease and patients, and significant efforts should be placed to identify effective symptomatic and systemic therapies and biomarkers for stratifying patients, predicting disease activity/severity, and response to treatment.
- In conclusion, although it is still considered a non-curable disease, the classification and innovative methods of Sjogren’s syndrome have been researched and developed over the past few years, and further diagnosis, assessment, and treatment are underway. Various potential novel medicines are in the pipeline to treat the disease.
- In November 2024, Johnson & Johnson announced that the U.S. FDA has granted Breakthrough Therapy designation (BTD) to nipocalimab for the treatment of adults with moderate-to-severe Sjögren’s disease (SjD), a chronic autoimmune condition with no approved advanced treatments.
Sjogren’s syndrome Market Report Summary
- The Sjogren’s syndrome market report offers extensive knowledge regarding the epidemiology segments and predictions, presenting a deep understanding of the potential future growth in diagnosis rates, disease progression, and treatment guidelines. It provides comprehensive insights into these aspects, enabling a thorough assessment of the subject matter.
- Additionally, an all-inclusive account of the current management techniques and emerging therapies and the elaborative profiles of late-stage (Phase III and Phase II) and prominent therapies that would impact the current treatment landscape and result in an overall Sjogren’s syndrome market shift has been provided in the report.
- The report also encompasses a comprehensive analysis of the Sjogren’s syndrome market, providing an in-depth examination of its historical and projected market size (2020–2034). It also includes the market share of therapies, detailed assumptions, and the underlying rationale for our methodology. The report also includes drug outreach coverage in the 7MM region.
- The Sjogren’s syndrome market report includes qualitative insights that provide an edge while developing business strategies by understanding trends through SWOT analysis and expert insights/KOL views, including experts from various hospitals and prominent universities, patient journey, and treatment preferences that help shape and drive the 7MM Sjogren’s syndrome market.
Key Factors Driving Sjogren's Syndrome Market
High Sjögren’s Syndrome Prevalence in the US
In 2024, the US recorded 1.5 million mild cases and ~265K moderate to severe cases of Sjögren’s syndrome. These cases are expected to rise during the forecast period, supported by improvements in disease awareness and diagnosis.
Sjögren’s Syndrome Established Therapies
Local treatments include pilocarpine, cevimeline, topical fluoride, cyclosporine, and autologous serum eye drops. Systemic therapies involve corticosteroids, hydroxychloroquine, and immunosuppressants such as methotrexate, cyclosporine A, azathioprine, leflunomide, and mycophenolic acid. Rituximab is widely used in biological therapy, with TNF-alpha inhibitors and BAFF inhibitors under development. Currently, the only approved drugs for complications of Sjögren’s syndrome are SALAGEN (pilocarpine; Eisai) and EVOXAC (cevimeline; Daiichi Sankyo), both indicated for dry mouth management.
Competitive Landscape and Emerging Therapies
Several novel therapies are in late-stage clinical development to address unmet needs in Sjögren’s syndrome. Key emerging candidates include ianalumab (VAY736; Novartis), SOTYKTU (deucravacitinib; Bristol Myers Squibb), and dazodalibep (VIB4920; Horizon Therapeutics/Amgen). These agents, with mechanisms targeting BAFF, TYK2, and CD40 pathways, have the potential to expand treatment options beyond symptomatic relief and reshape the market landscape.
DelveInsight's "Sjogren's Syndrome Market Insights, Epidemiology, and Market Forecast-2034" report delivers an in-depth understanding of the Sjogren's Syndrome, historical and forecasted epidemiology as well as the Sjogren's Syndrome therapeutics market trends in the United States, EU5 (Germany, Spain, Italy, France, and United Kingdom) and Japan.
The table given below further depicts the key segments provided in the report:
|
Study Period |
2020 to 2034 |
|
Forecast Period |
2024-2034 |
|
Geographies Covered |
|
|
Sjogren's Syndrome Market |
|
|
Sjogren's Syndromes Market Size | |
|
Sjogren's Syndrome Companies |
Advanz Pharma, Daiichi Sankyo, Resolve Therapeutics, GlaxoSmithKline, VIELABIO, RemeGen, Bristol Myers Squibb, TearSolutions, Pfizer Inc., AstraZeneca plc, Novartis AG, Sanofi S.A., Roche Holding AG, Johnson & Johnson, AbbVie Inc., Eli Lilly and Company, Biogen Inc., Amgen Inc., Celgene Corporation, Genentech, Inc, Boehringer Ingelheim International GmbH, Takeda Pharmaceutical Company Limited, Gilead Sciences, Inc., Merck & Co., Inc., Vertex Pharmaceuticals Incorporated, Bayer AG, and Many Others. |
|
Sjogren's Syndrome Epidemiology Segmentation |
|
Sjogren’s Syndrome Treatment Market
Sjogren’s Syndrome Overview
Sjogren’s syndrome is a chronic autoimmune disease with a specific predisposition for causing inflammation of the exocrine glands, predominantly the salivary and lacrimal glands, including the nose, upper respiratory tract, and oropharynx and, in women, the vagina. In Sjogren’s syndrome, the immune system inappropriately attacks various cells leading to activation of the various pathways, resulting in inflammation. The main consequence of this inflammation is the development of sicca symptoms due to damage to salivary and tear glands, such as dryness of the mucosal surfaces, principally in the mouth and eyes.
Five features of this disease, such as the development of sicca symptoms, systemic disease involvement, lymphocytic infiltration to exocrine glands, the presence of autoantibodies, and the increased risk of lymphoma in Sjogren’s syndrome patients, characterized Sjogren’s syndrome in the early years of its discovery. The autoimmune etiopathogenetic basis was confirmed in the 1960s, and the presence of the autoantibodies Sjogren’s syndrome-related antigen A (SSA; also known as anti-Ro/SSA antibodies) and SSB (also known as anti-La/SSB antibodies), in addition to organ-specific lymphocyte infiltration (for example, focal lymphocytic sialadenitis (FLS) or inflammation of the salivary glands) became central for the pathobiology and diagnosis of this disease. Since then, knowledge of Sjogren’s syndrome has progressed substantially.
Further details are provided in the report…
Sjogren’s syndrome Diagnosis
Sjogren’s syndrome is a multisystem disorder that is heterogeneous in its presentation, course, and outcome. There is still no single clinical, laboratory, pathological, or radiological feature that could serve as a gold standard for the diagnosis and/or classification of this syndrome. Labial salivary gland biopsy with a subsequent histopathological evaluation is currently in use.
The two main oral tests to evaluate salivary gland function are the measurement of salivary flow rates and salivary gland scintigraphy. The main ocular tests include Schirmer’s tests and analysis of the corneal surface using dyes (fluorescein and lissamine green) that stain degenerated or dead cells (corneal staining).
Ultrasonography and MRI are mainly used to evaluate the most common complications of Sjogren’s syndrome, such as infections and lymphoma.
Serologic and laboratory findings associated with Sjogren’s syndrome include diffuse hypergammaglobulinemia, which is found in approximately most of the patients with the disease. Serum levels of C-reactive protein are usually normal in patients with Sjogren’s syndrome, so increased levels indicate the presence of an infection. Raised serum levels of ß2-microglobulin have been reported in one-third of patients with Sjogren’s syndrome. Other laboratory abnormalities might suggest Sjogren’s syndrome-related renal involvement (such as increased levels of creatinine, proteinuria, hypokalemia, and a urine pH of >5.5 despite severe academia) or associated liver disease (indicated by increased levels of transaminases or alkaline phosphatases).
Further details related to country-based variations are provided in the report…
Sjogren’s Syndrome Treatment
There is no known cure for Sjogren’s syndrome, nor is there a specific treatment to restore gland secretion. Unfortunately, irreparable glandular damage often occurs at the time of sicca development, and therefore return of glandular function is not anticipated. Treatment is aimed at slowing or preventing further glandular damage, alleviating symptoms of dryness, and considering immune modulation in the case of extra-glandular disease.
Hydroxychloroquine is used as a first-line treatment for general symptoms, including sicca, arthralgia, and musculoskeletal pain. Methotrexate can be used in cases in which hydroxychloroquine is ineffective. Glucocorticoids are commonly used for episodes of salivary gland swelling as well as for more severe manifestations, including severe cutaneous, pulmonary, renal, musculoskeletal, or neurological involvement. When longer-term immune modulation is indicated, DMARDs (mycophenolate mofetil, cyclosporine A, azathioprine) are commonly used to limit glucocorticoid toxicity. The use of biologicals such as rituximab is indicated for severe disease with proven organ damage that is resistant to non-biologic immunosuppressant drugs or when conventional therapies are insufficient. Rituximab should be considered as a treatment option for adults with pSS and any of the following manifestations: vasculitis, severe parotid swelling, inflammatory arthritis, pulmonary disease, or peripheral neuropathy, especially mononeuritis multiplex.
Drugs with a systemic effect like secretagogues, such as pilocarpine and cevimeline, can be used for the symptomatic management of dry mouth as they stimulate the salivary glands.
Ocular dryness is addressed by limiting tear loss (wearing glasses, avoiding dry or windy environments), avoiding medications that may precipitate decreased tear production, using interventions to increase tear production with medication (e.g., cyclosporine, immune suppression) or punctual plugs, and replacement with artificial tears and lubrication.
Further details related to treatment and management are provided in the report…
Sjogren’s Syndrome Recent Developments
- In March 2025, Johnson & Johnson announced that the FDA granted Fast Track designation to investigational nipocalimab for treating adult patients with moderate-to-severe Sjögren’s disease (SjD), after receiving Breakthrough Therapy designation late last year. No advanced therapies are currently approved for this disease.
- In November 11, 2024, Johnson & Johnson announced that the U.S. FDA has granted Breakthrough Therapy designation (BTD) to nipocalimab for the treatment of adults with moderate-to-severe Sjögren’s disease (SjD), a chronic autoimmune condition with no approved advanced treatments.
Sjogren’s Syndrome Drug Chapters
The section dedicated to drugs in the Sjogren’s syndrome market report provides an in-depth evaluation of late-stage pipeline drugs (Phase III and Phase II) related to Sjogren’s syndrome.
The drug chapters section provides valuable information on various aspects related to clinical trials of Sjogren’s syndrome, such as the pharmacological mechanisms of the drugs involved, designations, approval status, patent information, and a comprehensive analysis of the pros and cons associated with each drug. Furthermore, it presents the most recent news updates and press releases on drugs targeting Sjogren’s syndrome.
Marketed Sjogren’s syndrome Therapies
SALAGEN (pilocarpine): ADVANZ Pharma
SALAGEN (pilocarpine) tablets are made from the naturally occurring alkaloid pilocarpine obtained from the leaflets of the South American shrub Pilocarpus jaborandi. Pilocarpine HCl is a cholinomimetic (cholinergic parasympathomimetic) agent capable of exerting a broad spectrum of pharmacologic effects with predominant muscarinic action. SALAGEN indications include the treatment of symptoms of dry mouth from salivary gland hypofunction caused by radiotherapy for cancer of the head and neck and the treatment of symptoms of dry mouth in patients with Sjogren’s syndrome.
EVOXAC (cevimeline): Daiichi Sankyo
EVOXAC (cevimeline) is a cholinergic agonist which binds to muscarinic receptors. Muscarinic agonists in enough dosage can increase the secretion of exocrine glands, such as salivary and sweat glands, and increase the tone of the smooth muscle in the gastrointestinal and urinary tracts. EVOXAC was developed by Daiichi Sankyo and is approved in the US, Europe, and Japan to treat patients with symptoms of dry mouth in patients with Sjogren’s syndrome. In January 2022, Daiichi Sankyo entered into an agreement with Cosette Pharmaceuticals; Daiichi Sankyo divested, and Cosette acquired rights for manufacturing, commercialization, and certain other rights for EVOXAC (cevimeline HCL) in the US.
Note: Detailed assessment will be provided in the final report of Sjogren’s syndrome…
Emerging Sjogren’s syndrome Therapies
CFZ 533 (iscalimab): Novartis
CFZ 533 (iscalimab) is a novel, fully human IGg1 anti-CD40 monoclonal antibody, preventing cluster of differentiation (CD40) pathway signaling and activation of CD40+ cell types. Currently, it is being evaluated in a Phase II clinical trial to treat patients with Sjogren’s syndrome. Apart from this indication, the company is also developing this molecule to treat patients with generalized myasthenia gravis, grave disease, rheumatoid arthritis, lupus nephritis, and renal transplant rejection.
VIB4920 (dazodalibep): Amgen
Dazodalibep is a CD40 ligand antagonist that blocks T-cell interaction with CD40-expressing B cells, disrupting the overactivation of the CD40 ligand co-stimulatory pathway. Currently, Amgen is in the recruiting phase for its Phase III clinical development of dazodalibep, an investigational medicine for the treatment of Sjogren’s syndrome. In November 2023, Amgen announced promising results from its Phase II study of dazodalibep for treating Sjogren’s syndrome.
Nipocalimab (IMAAVY)
Nipocalimab (IMAAVY) is a fully human IgG1 lambda monoclonal antibody that blocks the neonatal Fc receptor (FcRn), reducing circulating IgG and pathogenic autoantibodies. Approved by the FDA in April 2025 for AChR- or MuSK-positive generalized myasthenia gravis in adults and adolescents aged 12+, it features an aglycosylated Fc region without effector functions and is administered intravenously.
Sjogren’s Syndrome Market Outlook
At present, the therapeutic Sjogren’s syndrome market size in the US is mainly accounted for by local therapies, systemic therapy, and biological therapies. The local therapies mainly include pilocarpine, cevimeline, topical fluoride, topical cyclosporine, autologous serum eye drops, and others. Systemic therapy mainly uses corticosteroids, hydroxychloroquine, and immunosuppressants. Methotrexate (MTX), Cyclosporine A, Azathioprine, Leflunomide, and Mycophenolic acid are also commonly used DMARDs. Biological therapy with rituximab is in extensive use in the US market. Along with this, TNF-alpha inhibitors, BAFF, and others are in development.
Drugs with a systemic effect, secretagogues, such as pilocarpine and cevimeline, are approved by the US FDA for the treatment of dry eyes and mouth in patients with Sjogren’s syndrome. Both drugs are muscarinic receptor agonists and induce a transient increase in salivary and lacrimal gland outputs in patients with some residual functional glandular tissue. Common adverse effects of these drugs include sweating, flushing, the urgent need for urination, and gastrointestinal discomfort, which might limit their clinical application.
Despite all these available symptomatic treatments, Sjogren’s disease (SS) is among the most difficult to evaluate and manage. Clinicians are frequently challenged to differentiate symptoms related to disease activity. Additionally, the presence of multiple comorbidities may influence the severity and further complicate the evaluation process. There is a vital need for developing SS-specific outcome measures that encompass the spectrum of organ system involvement and are sensitive to clinically meaningful change. Better staging to identify patients with early disease and the discovery of novel biomarkers and/or genetic profiling to define specific patient subsets should facilitate better patient selection for targeted therapies.
To meet the current demands of the patient pool and to counter the unmet needs of the therapeutic market, drug developers are gradually shifting their attention toward SS as a possible indication for new targeted therapies. Several companies are working robustly on many new therapies, such as RSLV-132 (Resolve Therapeutics), VAY736 (Novartis/MorphoSys), CFZ533 (Novartis), OXERVATE (Dompe Farmaceutici), VIB4920 (Amgen), and SOTYKTU (Bristol Myers Squibb). A mid-stage pipeline is crowded, with several potential therapies with the imminent attention of big pharmaceutical companies for this market space. These all are anticipated to enter Sjogren’s syndrome market by 2030.
Further details are provided in the report…
Sjogren’s syndrome Market Insights
- Various key players are leading the treatment landscape of Sjogren’s syndrome, such as Novartis, Amgen, Resolve Therapeutics, Bristol Myers Squibb, and others. The details of the country-wise and therapy-wise Sjogren’s syndrome Market size have been provided below.
- In 2023, the total Sjogren’s syndrome Market size was around USD 1,900 million, which is expected to increase by 2034 during the study period (2020–2034) in the 7MM.
- Among the 7MM, the United States accounted for the highest Sjogren’s syndrome Market size in 2023, whereas Japan accounted for the least Sjogren’s syndrome Market size.
- Among the EU4 and the UK, the UK had the largest Sjogren’s syndrome Market size, while the lowest Sjogren’s syndrome Market was estimated to be covered by Spain in 2023.
- Germany accounted for approximately 9% of the total Sjogren’s syndrome Market size among the 7MM.
- Novartis and Amgen are key players in the Sjogren’s syndrome Market, and their drugs, CFZ533 (iscalimab) and VIB4920 (dazodalibep) are expected to generate the highest revenue in the 7MM by 2034.
Sjogren’s Syndrome Epidemiology
The Sjogren’s syndrome epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by diagnosed prevalent cases, type-specific cases, gender-specific cases, severity-specific cases, antigen-specific cases, total treated cases of Sjogren’s syndrome in the United States, EU4 countries (Germany, France, Italy, Spain) and the United Kingdom, and Japan from 2020 to 2034.
- In the US, nearly 1,500,000 diagnosed prevalent cases of Sjogren’s syndrome were observed in the year 2023, which is expected to increase during the forecast period (2024–2034).
- In the US, the highest number of antigen-specific cases accounted for auto-antibodies (+ve), followed by anti-Ro/SSA (+ve) and anti-La/SSB (+ve) in 2023.
- As per DelveInsight’s analysis, a higher number of cases were observed for mild than moderate to severe, based on severity-specific cases of Sjogren’s syndrome, throughout the 7MM.
- Among EU4 and the UK, the majority of Sjogren’s syndrome cases were reported in females. The highest number of gender-specific cases was observed in the United Kingdom in 2023.
- In 2023, the US accounted for the highest number of treated cases, whereas Japan had the lowest number of treated cases. In Japan, there were around 54,000 treated cases of Sjogren’s syndrome in 2023. These cases are likely to rise by 2034.
KOL Views
To stay abreast of the latest trends in the Sjogren’s syndrome market, we conduct primary research by seeking the opinions of Key Opinion Leaders (KOLs) and Subject Matter Experts (SMEs) who work in the relevant field. This helps us fill any gaps in data and validate our secondary research.
We have reached out to industry experts to gather insights on various aspects of Sjogren’s syndrome, including the evolving treatment landscape, patients’ reliance on conventional therapies, their acceptance of therapy switching, drug uptake, and challenges related to accessibility. The experts we contacted included medical/scientific writers, professors, and researchers from prestigious universities in the US, Europe, the UK, and Japan.
Our team of analysts at DelveInsight connected with more than 15 KOLs across the 7MM. We contacted institutions such as the John Hopkins Medicine, Duke University, University of Pennsylvania, University of Munich, etc., among others. By obtaining the opinions of these experts, we gained a better understanding of the current and emerging treatment patterns in the Sjogren’s syndrome market, which will assist our clients in analyzing the overall epidemiology and market scenario.
Qualitative Analysis
We perform Qualitative and Market Intelligence analysis using various approaches, such as SWOT analysis and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the Analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving treatment landscape.
Conjoint Analysis analyzes multiple approved and emerging Sjogren’s syndrome therapies based on relevant attributes such as safety, efficacy, frequency of administration, designation, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy.
In efficacy, the trial’s primary and secondary outcome measures are evaluated; for instance, in trials for Sjogren’s syndrome, important primary and secondary endpoints are ESSDAI score, ESSPRI scores, FACIT-F score, Profile of Fatigue Score (ProF), etc. Based on these parameters, the overall efficacy is evaluated.
Further, the therapies’ safety is evaluated wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the route of administration, order of entry and designation, probability of success, and the addressable patient pool for each therapy. According to these parameters, a final weightage score is decided based on which the emerging therapies are ranked.
Sjogren’s syndrome Market Access and Reimbursement
Because newly authorized drugs are often expensive, some patients escape receiving proper treatment or use off-label, less expensive prescriptions. Reimbursement plays a critical role in how innovative treatments can enter the market. The cost of the medicine, compared to the benefit it provides to patients who are being treated, sometimes determines whether or not it will be reimbursed. Regulatory status, target population size, the setting of treatment, unmet needs, the number of incremental benefit claims, and prices can all affect market access and reimbursement possibilities.
The Sjogren’s syndrome market report further provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of approved therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Sjogren’s Syndrome Market Report Insights
- Sjogren’s syndrome Patient Population
- Sjogren’s Syndrome Therapeutic Approaches
- Sjogren’s Syndrome Market Size
- Sjogren’s syndrome Market Trends
- Existing Sjogren’s syndrome Market Opportunity
- Sjogren’s syndrome Drugs Market
Sjogren’s Syndrome Market Report Key Strengths
- Eleven-year Forecast
- The 7MM Coverage
- Sjogren’s Syndrome Epidemiology Segmentation
- Key Cross Competition
Sjogren’s Syndrome Market Report Assessment
- Current Sjogren’s SyndromeTreatment Practices
- Sjogren’s Syndrome Market Reimbursements
- Sjogren’s syndrome Market Attractiveness
- Qualitative Analysis (SWOT, Conjoint Analysis, Unmet needs)
- Sjogren’s Syndrome Market Drivers
- Sjogren’s Syndrome Market Barriers
Key Questions Answered In The Sjogren’s syndrome Market Forecast Report
- Would there be any changes observed in the current treatment approach?
- Will there be any improvements in Sjogren’s syndrome management recommendations?
- Would research and development advances pave the way for future tests and therapies for Sjogren’s syndrome?
- Would the diagnostic testing space experience a significant impact and lead to a positive shift in the treatment landscape of Sjogren’s syndrome?
- What kind of uptake will the new therapies witness in the coming years in Sjogren’s syndrome patients?