Adrenoleukodystrophy Market Summary
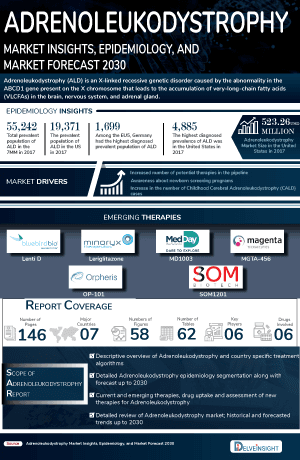
- In 2023, the United States accounted for the largest Adrenoleukodystrophy market size (~USD 400 Million) in comparison to EU4 (Germany, Spain, Italy, France), the United Kingdom, and Japan.
- Adrenoleukodystrophy (ALD) is an X-linked disorder resulting from a defect in peroxisomal beta-oxidation of very-long-chain fatty acids (VLCFA).
- Based on the age of onset and severity of its symptoms, ALD is classified into three main types: childhood cerebral adrenoleukodystrophy (CALD), adrenomyeloneuropathy (AMN), and Addison's disease.
Adrenoleukodystrophy Market and Epidemiology Analysis
- According to DelveInsight's estimates, CALD accounted for the largest share (~50%) of diagnosed ALD cases in the United States in 2023.
- Adrenoleukodystrophy (ALD) is a rare genetic disorder that predominantly affects male during childhood. In 2023, around 60% of ALD cases were diagnosed in male based on the gender-specific prevalence of the disease in the 7MM.
- At present, there is no cure for Adrenoleukodystrophy. One of the effective treatment option for cerebral Adrenoleukodystrophy is a stem cell transplant, a procedure in which the patient receives blood stem cells from a genetically matched donor. Hematopoietic stem cell therapy is effective in X-Adrenoleukodystrophy if initiated early, but treatment for X-AMN is lacking
- The Adrenoleukodystrophy market size in the 7MM is expected to increase due to increasing awareness of newborn screening programs, growing number of cases of cerebral adrenoleukodystrophy (CALD), and expected approval of potential Adrenoleukodystrophy therapies over the forecast period (2024-2034).
- In September 2022, Bluebird Bio received the US FDA accelerated approval for SKYSONA gene therapy for early, active cerebral adrenoleukodystrophy (CALD).
- Several emerging Adrenoleukodystrophy therapies like Leriglitazone (MIN-102), PXL770, and others are in development for Adrenoleukodystrophy to address the existing unmet medical needs in the Adrenoleukodystrophy treatment market.
- In January 2024, Minoryx Therapeutics announced that the EMA’s Committee for Medicinal Products for Human Use (CHMP) has recommended not to grant marketing authorization for Nezglyal (leriglitazone) as a treatment of X-Adrenoleukodystrophy .
Factors Impacting the Adrenoleukodystrophy Treatment Market Growth
Increasing Disease Awareness:
Better recognition of ALD symptoms among clinicians and patients is improving diagnosis rates, expanding the treated population.
Advancements in Diagnostic Technologies:
Newborn screening programs and improved genetic testing enable earlier detection and intervention, broadening the target market.
Innovation in Therapeutics:
Development of novel treatment approaches (e.g., gene therapy, enzyme replacement, small molecules) is driving market optimism and investment.
Favorable Regulatory Support:
Orphan drug designations, priority reviews, and accelerated approval pathways encourage pharmaceutical innovation in rare diseases like ALD.
Rising R&D Investments:
Increased funding from biopharma companies and research institutions accelerates clinical development pipelines.
Collaborations and Partnerships:
Strategic alliances between biotech firms, academic centers, and patient advocacy groups speed up translational research and market entry.
Improved Patient Support Networks:
The growth of advocacy organizations helps with patient recruitment for clinical trials and supports long-term disease management.
DelveInsight's “Adrenoleukodystrophy Market Insights, Epidemiology and Market Forecast – 2034” report delivers an in-depth understanding of adrenoleukodystrophy, historical and forecasted epidemiology as well as the adrenoleukodystrophy market trends in the United States, EU4 (Germany, Spain, Italy, and France) and the United Kingdom, and Japan.
Adrenoleukodystrophy market report provides real-world prescription pattern analysis, emerging drugs, market share of individual therapies, and historical and forecasted 7MM adrenoleukodystrophy market size from 2020 to 2034. The Adrenoleukodystrophy treatment report also covers current adrenoleukodystrophy treatment practices/algorithms and unmet medical needs to curate the best opportunities and assess the market’s underlying potential.
Scope of the Adrenoleukodystrophy Market Report | |
|
Study Period |
2020-2034 |
|
Forecast Period |
2024-2034 |
|
Geographies Covered |
US, EU4 (Germany, France, Italy, and Spain) and the UK, and Japan |
|
Adrenoleukodystrophy Epidemiology |
|
|
Adrenoleukodystrophy Market |
|
|
Adrenoleukodystrophy Market Analysis |
|
|
Adrenoleukodystrophy Companies |
|
Adrenoleukodystrophy Treatment Understanding
Adrenoleukodystrophy Overview, Country-Specific Treatment Guidelines and Diagnosis
ALD is caused by mutations in the ABCD1 gene that prevent the body from breaking down very-long-chain fatty acids (VLCFAs). As a result, VLCFAs build up in the brain, nervous system, and adrenal glands. It is proposed that the presence of VLCFA in myelin induces myelin instability, which results in an immune-mediated process in which presentation of a lipid antigen may result in substantial myelin destruction. It is a progressive disorder of young males associated with elevated levels of very-long-chain fatty acids, due to defective β-oxidation and ABCD1 gene mutations encoding peroxisomal membrane protein X-ALD protein.
Diagnosing adrenoleukodystrophy (ALD) involves initially measuring VLCFA levels through a blood test. Elevated VLCFA levels suggest a potential ALD diagnosis. To confirm the diagnosis, a genetic test is typically ordered to identify mutations in the ABCD1 gene, which is associated with ALD. Other diagnostic tests for adrenoleukodystrophy (ALD) may include brain imaging (MRI), adrenal function tests, neurological evaluation, hormone testing, lipid panel, and sensory assessments to aid in diagnosis and assessment of disease progression.
The adrenoleukodystrophy treatment market report provides an overview of adrenoleukodystrophy pathophysiology, diagnostic approaches, and detailed treatment algorithm along with a real-world scenario of a patient’s journey beginning from the first symptom, the time taken for diagnosis to the entire treatment process.
Further details related to country-based variations in diagnosis are provided in the report...
Adrenoleukodystrophy Treatment
General supportive care and symptomatic treatment for patient and family, provided by pediatrician or neurologist, with appropriate specialist consultation, nursing, schools, rehabilitation, and social agencies, are the cornerstones for the care and treatment of patients with X-ALD. It is primarily managed by hormone replacement therapy, dietary therapy with Lorenzo’s oil, and hematopoietic stem cell transplantation.
SKYSONA (elivaldogene autotemcel) became the first FDA approved Adrenoleukodystrophy therapy shown to slow the progression of neurologic dysfunction in boys 4-17 years of age with early, active cerebral adrenoleukodystrophy (CALD).
Further details related to country-based variations in treatment are provided in the report...
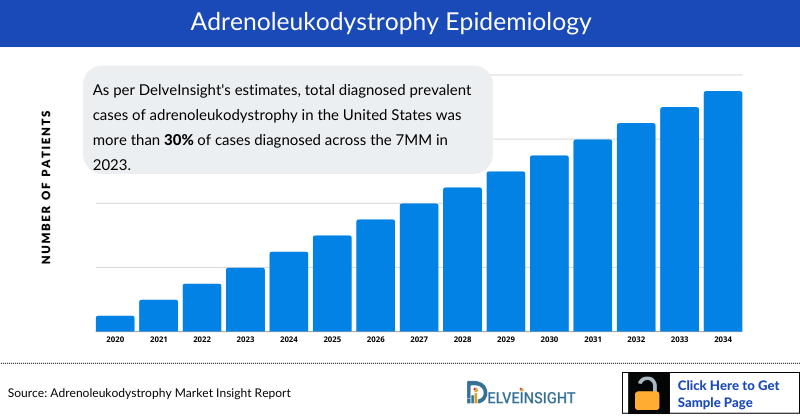
Adrenoleukodystrophy Epidemiology
The Adrenoleukodystrophy epidemiology chapter in the Adrenoleukodystrophy treatment market report provides historical as well as forecasted in the 7MM covering the United States, EU4 countries (Germany, France, Italy, and Spain), the United Kingdom, and Japan from 2024 to 2034. The adrenoleukodystrophy epidemiology is segmented with detailed insights into Total Prevalent Cases of Adrenoleukodystrophy (ALD), Total Diagnosed Prevalent Cases of Adrenoleukodystrophy (ALD), Gender-specific Diagnosed Prevalence of ALD, and Type-specific Diagnosed Prevalence of ALD.
Key Findings from Adrenoleukodystrophy Epidemiological Analyses and Forecast
- According to the findings, adrenoleukodystrophy in the 7MM was found to be more prevalent in male compared to female.
- As per DelveInsight's estimates, total diagnosed prevalent cases of adrenoleukodystrophy in the United States was more than 30% of cases diagnosed across the 7MM in 2023.
- Among the EU4 and the UK, the total prevalent population of adrenoleukodystrophy patients were the highest in Germany with approximately 25% cases, followed by France and UK in 2023.
- DelveInsight’s consultant estimates that the cerebral Adrenoleukodystrophy will contribute the maximum number of cases in the United States in 2023.
Adrenoleukodystrophy Epidemiology Segmentation
- Total Prevalent Cases of Adrenoleukodystrophy
- Total Diagnosed Prevalent Cases of Adrenoleukodystrophy
- Gender-specific Diagnosed Prevalence of ALD
- Type-specific Diagnosed Prevalence of ALD
Adrenoleukodystrophy Market Recent Developments and Breakthroughs
- In February 2025, Lam Research announced the launch of its next-generation Atomic Layer Deposition (ALD) equipment in South Korea, designed specifically to support high-precision thin-film deposition for 3D NAND volume production. This new system marks a significant materials shift by using molybdenum instead of tungsten, a change aimed at improving device performance, energy efficiency, and scalability. The introduction aligns with accelerating global demand for AI-optimized memory architectures, where lower resistance and improved film uniformity are essential for high-density 3D NAND stacks.
- In May 2025, Beneq revealed that its Transform ALD platform was qualified for high-volume manufacturing of GaN power devices by a major Asian semiconductor producer. The tool supports both plasma-enhanced ALD (PEALD) and thermal ALD, enabling manufacturers to achieve high-yield, scalable GaN device production with enhanced interface engineering and reliability. This qualification highlights Beneq’s growing role in next-generation power electronics, where GaN is rapidly expanding across electric mobility, consumer electronics, and industrial applications.
Adrenoleukodystrophy Drug Analysis
The drug chapter segment of the adrenoleukodystrophy drugs market report encloses a detailed analysis of adrenoleukodystrophy marketed drugs and late-stage (Phase III and Phase II) pipeline drugs. It also deep dives into the adrenoleukodystrophy clinical trial details, recent and expected market approvals, patent details, the latest news, and recent deals and collaborations.
Marketed Adrenoleukodystrophy Drugs
SKYSONA (elivaldogene autotemcel): Bluebird Bio
SKYSONA is an autologous hematopoietic stem cell-based gene therapy. In September 2022, the US FDA granted accelerated approval of SKYSONA (elivaldogene autotemcel), also known as eli-cel, to slow the progression of neurologic dysfunction in boys 4-17 years of age with early, active cerebral adrenoleukodystrophy (CALD). The approval of SKYSONA was based on data from Bluebird Bio’s Phase II/III study ALD-102 (Starbeam) (N=32) and Phase III ALD-104 (N=35) study. This indication is approved under accelerated approval based on 24-month major functional disability (MFD) free survival.
Emerging Adrenoleukodystrophy Drugs
Leriglitazone (MIN-102): Minoryx Therapeutics/Neuraxpharm
Leriglitazone is Minoryx Therapeutics’s novel orally bioavailable and selective PPAR gamma agonist with a potential first-in-class and best-in-class profile for CNS diseases. It has demonstrated brain penetration and a favorable safety profile. Leriglitazone showed clinical benefit in both adult X-ALD patients in ADVANCE and pediatric X-ALD patients in NEXUS trial. Data from ADVANCE showed that leriglitazone reduces the progression of lesions and the development of progressive cALD. Leriglitazone has been granted orphan drug status for X-ALD from the FDA and the EMA and Fast Track and Rare Pediatric Disease designation from the FDA for the treatment of X-ALD.
Minoryx Therapeutics and Neuraxpharm are seeking re-examination by the EMA's CHMP for conditional approval of leriglitazone as a treatment for cerebral adrenoleukodystrophy (cALD) after the initial marketing authorization recommendation was not granted.
PXL770: Poxel
PXL770 is a first-in-class direct AMPK activator. Clinical Phase I and IIa development has demonstrated target engagement and translation of several metabolic efficacy parameters to humans which suggests the likelihood of broader translation for this mechanism. In early 2022, the US FDA granted Fast Track and Orphan Drug Designation to PXL770 for the treatment of patients with adrenomyeloneuropathy.
A Phase IIa clinical POC biomarker studies of PXL770 is planned to initiate as soon as possible, subject to financing. The initial focus will be on ALD patients with adrenomyeloneuropathy, the largest subtype of Adrenoleukodystrophy.
Note: Detailed emerging therapies assessment will be provided in the final report...
Adrenoleukodystrophy Market Outlook
Adrenoleukodystrophy Companies, such as Bluebird Bio, Minoryx Therapeutics/Neuraxpharm, Poxel, and others are evaluating their lead candidates in different stages of clinical development. They aim to investigate their products for the treatment of adrenoleukodystrophy.
The United States dominates the Adrenoleukodystrophy market, constituting about 40% of the Adrenoleukodystrophy market share, surpassing the Adrenoleukodystrophy market presence of the EU4 (Germany, Spain, Italy, France), the United Kingdom, and Japan.
Leriglitazone (MIN-102) is expected to capture a significant Adrenoleukodystrophy market share during the forecast period.
The Adrenoleukodystrophy market growth is driven by a growing pipeline of potential therapies, heightened awareness of newborn screening initiatives, and a rising incidence of cerebral adrenoleukodystrophy (CALD) cases.
Adrenoleukodystrophy Drugs Uptake
This section focuses on the uptake rate of potential Adrenoleukodystrophy drugs expected to be launched in the Adrenoleukodystrophy treatment market during 2024–2034, which depends on the competitive landscape, safety, and efficacy data along with order of entry. It is important to understand that the key Adrenoleukodystrophy companies evaluating their novel therapies in the pivotal and confirmatory trials should remain vigilant when selecting appropriate comparators to stand the greatest chance of a positive opinion from regulatory bodies, leading to approval, smooth launch, and rapid uptake.
Further detailed analysis of emerging therapies drug uptake in the report...
Adrenoleukodystrophy Pipeline Development Activities
The Adrenoleukodystrophy treatment market report provides insights into different therapeutic candidates in Phase III and Phase II stages. It also analyzes key Adrenoleukodystrophy involved in developing targeted therapeutics.
Adrenoleukodystrophy Clinical Trial Activities
The Adrenoleukodystrophy treatment market report covers information on collaborations, acquisitions and mergers, licensing, and patent details for adrenoleukodystrophy emerging therapies.
Latest KOL Views on Adrenoleukodystrophy
To keep up with the real-world scenario in current and emerging Adrenoleukodystrophy market trends, we take opinions from Key Industry leaders working in the domain through primary research to fill the data gaps and validate our secondary research. Industry Experts were contacted for insights on the evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake along with challenges related to accessibility.
DelveInsight’s analysts connected with 10+ KOLs to gather insights; however, interviews were conducted with 5+ KOLs in the 7MM. Centers such as Albert Einstein College of Medicine, Penn State Hershey Medical Center, University Hospital Heidelberg, University of Minnesota, etc., were contacted. Their opinion helps understand and validate current and emerging treatment patterns of adrenoleukodystrophy. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
Adrenoleukodystrophy Report Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT analysis and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of gaps in disease diagnosis, patient awareness, physician acceptability, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided.
Conjoint Analysis analyzes multiple approved and emerging Adrenoleukodystrophy therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy.
In efficacy, the trial’s primary and secondary outcome measures are evaluated; one of the most important primary outcome measures is time to death and requirement for permanent ventilator support.
Further, the therapies’ safety is evaluated wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging Adrenoleukodystrophy therapies are decided.
Adrenoleukodystrophy Market Access and Reimbursement
Reimbursement of rare disease therapies can be limited due to lack of supporting policies and funding, challenges of high prices, lack of specific approaches to evaluating rare disease drugs given limited evidence, and payers’ concerns about budget impact. The high cost of rare disease drugs usually has a limited effect on the budget due to the small number of eligible patients being prescribed the drug. The US FDA has approved several rare disease therapies in recent years. From a patient perspective, health insurance and payer coverage guidelines surrounding rare disease treatments restrict broad access to these treatments, leaving only a small number of patients who can bypass insurance and pay for products independently.
The Adrenoleukodystrophy treatment market report further provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of currently used therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Scope of the Adrenoleukodystrophy Market Report
- The Adrenoleukodystrophy treatment market report covers a segment of key events, an executive summary, descriptive overview of adrenoleukodystrophy, explaining its causes, signs and symptoms, pathogenesis, and currently available therapies.
- Comprehensive insight has been provided into the epidemiology segments and forecasts, the future growth potential of diagnosis rate, and disease progression along with country specific treatment guidelines.
- Additionally, an all-inclusive account of both the current and emerging therapies, along with the elaborative profiles of late-stage and prominent therapies, will have an impact on the current treatment landscape.
- A detailed review of the adrenoleukodystrophy market, historical and forecasted market size, market share by therapies, detailed assumptions, and rationale behind our approach is included in the Adrenoleukodystrophy treatment market report, covering the 7MM drug outreach.
- The Adrenoleukodystrophy drugs market report provides an edge while developing business strategies, by understanding trends, through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help in shaping and driving the 7MM adrenoleukodystrophy treatment market.
Adrenoleukodystrophy Market Report Insights
- Adrenoleukodystrophy Patient Population
- Adrenoleukodystrophy Therapeutic Approaches
- Adrenoleukodystrophy Pipeline Analysis
- Adrenoleukodystrophy Market Size
- Adrenoleukodystrophy Market Trends
- Existing and Future Adrenoleukodystrophy Market Opportunity
Adrenoleukodystrophy Market Report Key Strengths
- Eleven-year Forecast
- 7MM Coverage
- Adrenoleukodystrophy Epidemiology Segmentation
- Inclusion of Country Specific Treatment Guidelines
- KOL’s Feedback On Approved and Emerging Adrenoleukodystrophy Therapies
- Key Cross Competition
- Conjoint Analysis
- Adrenoleukodystrophy Drugs Uptake
- Key Adrenoleukodystrophy Market Forecast Assumptions
Adrenoleukodystrophy Market Report Assessment
- Current Treatment Practices
- Unmet Needs
- Adrenoleukodystrophy Pipeline Product Profiles
- Adrenoleukodystrophy Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
FAQs
- What is the growth rate of the 7MM adrenoleukodystrophy treatment market?
- What was the adrenoleukodystrophy market size, the market size by therapies, market share (%) distribution in 2020, and what would it look like in 2034? What are the contributing factors/key catalysts for this growth?
- Is there any unexplored patient setting that can open the window for growth in the future?
- What are the pricing variations among different geographies for approved and off-label Adrenoleukodystrophy therapies?
- How would the market drivers, barriers, and future opportunities affect the Adrenoleukodystrophy market dynamics and subsequent analysis of the associated trends?
- What are the current and emerging options for the treatment of adrenoleukodystrophy?
- How many Adrenoleukodystrophy companies are developing therapies for the treatment of adrenoleukodystrophy?
- What are the recent novel therapies, targets, mechanisms of action, and technologies developed to overcome the limitations of existing therapies?
- Patient/physician acceptability in terms of preferred treatment options as per real-world scenarios?
- What are the country-specific accessibility issues of expensive, recently approved Adrenoleukodystrophy therapies?
Reasons to buy Adrenoleukodystrophy Report
- The Adrenoleukodystrophy treatment market report will help in developing business strategies by understanding the latest trends and changing treatment dynamics driving the adrenoleukodystrophy market.
- Insights on patient burden/ Adrenoleukodystrophy prevalence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years
- Understand the existing Adrenoleukodystrophy market opportunities in varying geographies and the growth potential over the coming years.
- Distribution of historical and current Adrenoleukodystrophy patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
- Identifying strong upcoming Adrenoleukodystrophy companies in the Adrenoleukodystrophy market will help devise strategies to help get ahead of competitors.
- Detailed analysis and ranking of class-wise potential current and emerging therapies under the conjoint analysis section to provide visibility around leading classes.
- Highlights of access and reimbursement policies of approved therapies, barriers to accessibility of expensive off-label therapies, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing Adrenoleukodystrophy market so that the upcoming Adrenoleukodystrophy companies can strengthen their development and launch strategy.



-pipeline.png&w=256&q=75)