Blogs
Healthcare and Medtech Research Reports
Articles
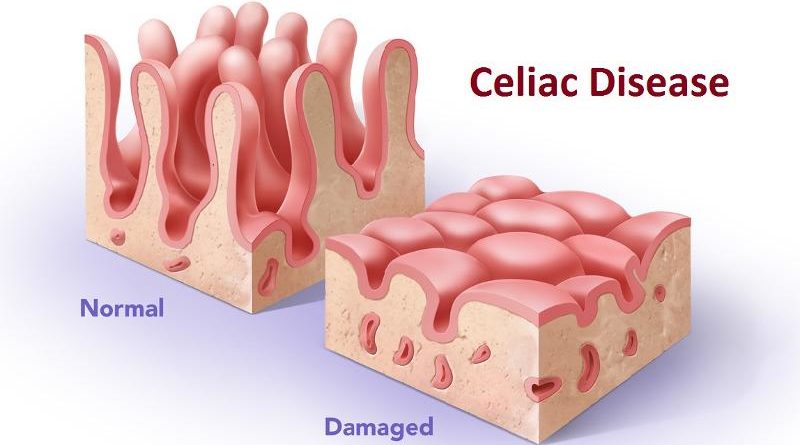
Celiac disease is a serious chronic, autoimmune disorder that can occur in genetically predisposed people. The ingestion of gluten (a protein found in wheat, rye and barley) by a person suffering from celiac disease leads to damage in the small intestine. Celiac disease can be difficult to diagnose because it affects people differently. There are about 300 known symptoms which may occur in the digestive system or other parts of the body. According to the latest report of DelveInsight, “Celiac Disease (CD) - Market Insights, Epidemiology & Market Forecast, 2025”, In 2016 total number of prevalent and diagnosed population of CD was found to be more than 3.9 Million ...
Explore More...