Anti- C-C Motif Chemokine Receptor 8 Antibodies Market Summary
- The anti-CCR8 antibodies market in the 7MM is projected to grow at a significant CAGR by 2040 in leading countries (US, EU4, UK and Japan).
Anti-CCR8 Antibodies Market and Epidemiology Analysis
- The anti-CCR8 antibodies market holds potential in addressing unmet needs across inflammatory and cancerous diseases. However, some setbacks in early-stage trials underscore the importance of strategic pipeline development and patient stratification to unlock commercial viability.
- CCR8 (Chemokine Receptor 8) is a G protein-coupled receptor (GPCR) that is part of the CC chemokine receptor family. It is essential for directing the movement of immune cells—such as T cells, dendritic cells (DCs), monocytes, and innate lymphoid cells (ILCs)—toward sites of inflammation or infection.
- CCR8 and its ligand CCL1 play an important role in regulating the recruitment and function of regulatory T cells (Tregs) within the tumor microenvironment (TME).
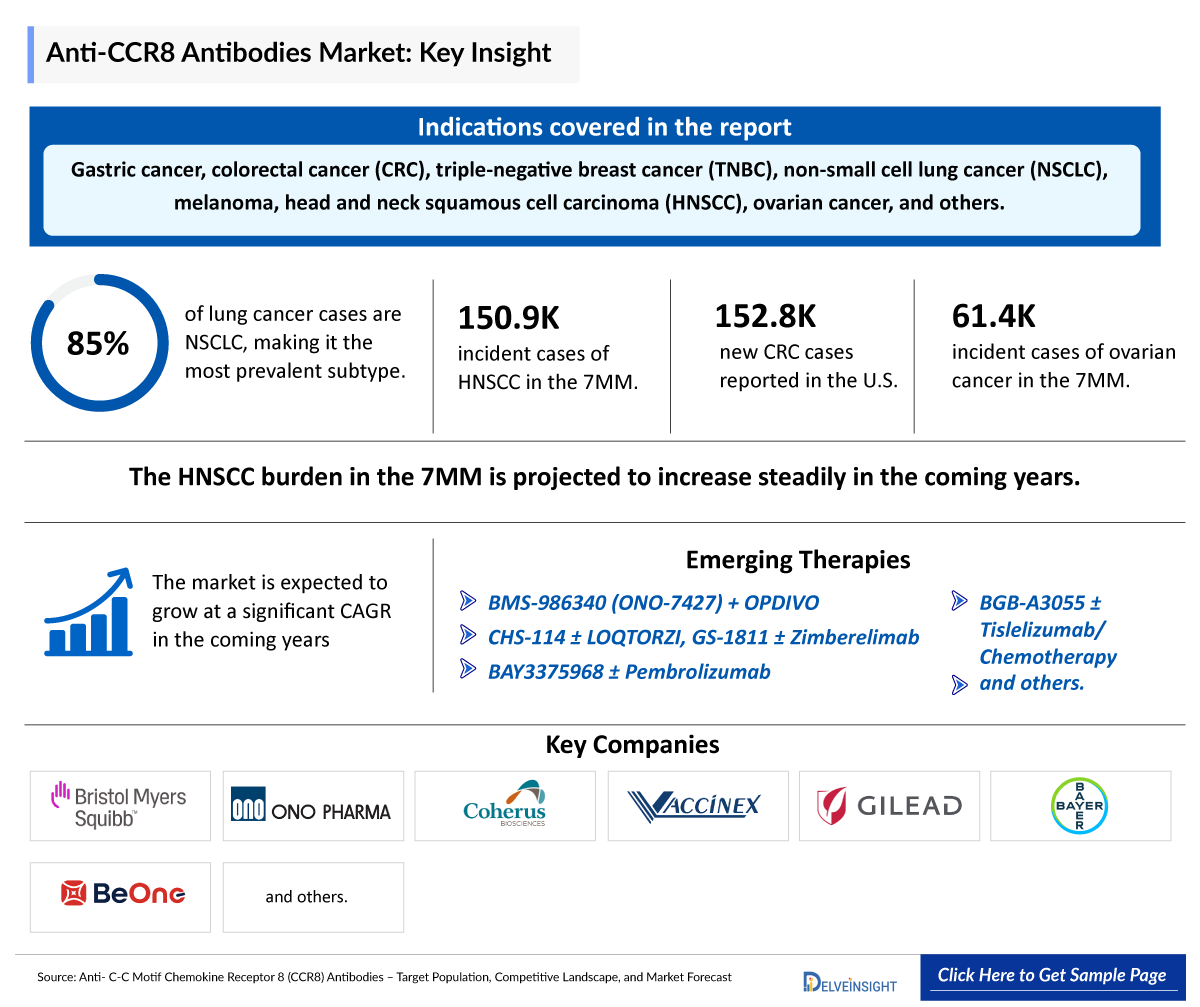
- Anti-CCR8 antibodies are emerging immunotherapeutic candidates with potential applications in cancer treatment—including gastric cancer, colorectal cancer (CRC), triple-negative breast cancer (TNBC), non-small cell lung cancer (NSCLC), melanoma, head and neck squamous cell carcinoma (HNSCC), ovarian cancer, and others. They are being investigated for their ability to selectively deplete tumor-infiltrating Tregs. Research also suggests possible applications in autoimmune and inflammatory diseases in future.
- CCR8 expression may serve as a biomarker for identifying tumor-infiltrating Tregs and various inflammatory conditions.
- High-quality monoclonal antibodies can be employed for tissue staining, biopsy evaluation, and immune cell profiling, particularly in the contexts of cancer immunotherapy and allergy diagnostics.
- As of May, there are no anti-CCR8 antibodies that have received regulatory approval for commercial use by major health authorities, including the US Food and Drug Administration (FDA), the European Medicines Agency (EMA).
- The emerging pipeline of anti-CCR8 antibodies includes BMS-986340 (ONO-7427) + OPDIVO (BMS/Ono Pharma), CHS-114 ± LOQTORZI (Coherus Biosciences and Vaccinex), GS-1811 ± Zimberelimab (Gilead Sciences), BAY3375968 ± Pembrolizumab (Bayer), and BGB-A3055 ± Tislelizumab/Chemotherapy (BeOne Medicines, formerly BeiGene), and others.
- Overall, this is an exciting class that holds great potential for the development of treatment for autoimmune disorders and other indications as well. The maturation of current studies over the next few years will lead to a better understanding of anti-CCR8 antibodies and define their role in the therapy of autoimmune indications, cancer indications, and others.
DelveInsight’s “Anti- C-C Motif Chemokine Receptor 8 (CCR8) Antibodies Target Population, Competitive Landscape, and Market Forecast – 2040” report delivers an in-depth understanding of the anti-CCR8 antibodies, historical and competitive landscape as well as its Anti- C-C Motif Chemokine Receptor 8 Antibodies therapeutics market trends in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
The anti-CCR8 market report provides current treatment practices, emerging therapies, market share of individual therapies, and current and forecasted anti-CCR8 market size in 7MM from 2020 to 2040. The report also covers current anti-CCR8 treatment practices/algorithms and unmet medical needs to curate the best opportunities and assess the market’s potential.
|
Study Period |
2020–2040 |
|
Forecast Period |
2025–2040 |
|
Geographies Covered |
US, EU4 (Germany, France, Italy, and Spain) the UK, and Japan |
|
Anti-CCR8 Antibodies Epidemiology |
Segmented by:
|
|
Anti-CCR8 Antibodies Companies |
|
|
Anti-CCR8 Antibodies Therapies |
|
|
Anti-CCR8 Antibodies Market Segmentation |
Segmented by:
|
|
Anti-CCR8 Antibodies Market Analysis |
|
Anti- C-C Motif Chemokine Receptor 8 Antibodies Treatment Market
Anti- C-C Motif Chemokine Receptor 8 Antibodies Overview
C-C motif chemokine receptor 8 (CCR8) is a class A GPCR that is predominantly and selectively expressed on Treg cells within tumors. These Treg cells suppress anti-tumor immune responses by inhibiting effector T cell activity. Elevated levels of intratumoral Tregs are associated with worse clinical outcomes and poorer prognoses across various cancer types. This has led to the hypothesis that targeting and selectively depleting these Treg cells could restore anti-tumor immunity and enhance the effectiveness of cancer immunotherapy. Supporting this, recent preclinical studies in mice have shown that using an anti-CCR8 antibody to eliminate Treg cells can elicit potent anti-tumor effects. CCR8 belongs to the ten-member subfamily. It is activated by its natural ligand, CCL1 (C-C motif chemokine ligand 1), and signals through the inhibitory G protein Gi. However, the detailed molecular structure of CCR8 and the precise mechanisms underlying its activation are still not fully understood. Antibodies targeting GPCRs hold great promise for therapeutic use, in particular for protein ligand GPCRs such as chemokine receptors that may be more challenging to drug with small molecules.
Further details related to country-based variations are provided in the report...
Anti- C-C Motif Chemokine Receptor 8 Antibodies Epidemiology
The anti-CCR8 antibodies epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented as total cases in selected indications for anti-CCR8 antibodies, total eligible patient pool in selected indications for anti-CCR8 antibodies, and total treated cases in selected indications for anti-CCR8 antibodies in the 7MM covering the United States, EU4 (Germany, France, Italy, and Spain), and the United Kingdom, and Japan from 2020 to 2040.
- Among lung cancer cases, NSCLC accounts for approximately 85% of all diagnoses. This distribution highlights that NSCLC prevalance is highest among lung cancer.
- According to the American Cancer Society (2024), an estimated 152,810 new cases of CRC was reported in 2024, with 81,540 cases in men and 71,270 cases in women in the US.
- As per estimates, total incident cases of ovarian cancer in the 7MM were ~61,400 in 2024.
- Among the EU4 and the UK, Germany had the highest number of ovarian cancer cases, while Spain had the lowest number in 2024.
- In 2024, the total incident cases of NSCLC in 7MM was ~ 537,680, out of which the contribution of the US was ~38%.
- The total number of incident cases of gastric cancer (including GEJ) in the 7MM accounted for approximately 211,550 in 2024. These cases are expected to increase by 2040.
- The total incident cases of HNSCC in the 7MM were approximately 150,900 in 2024, which is expected to increase in the upcoming years.
- In 2024, the American Cancer Society estimates approximately 310,720 new cases of invasive breast cancer among women in the US. TNBC patient pool represents 15% to 20% of all primary breast cancers and is the most aggressive subtype of breast cancer.
Target Pool Assessment of Anti-CCR8 Antibodies | |
|
Indication |
Estimated Incident Cases in the US (2024) |
|
Gastric cancer |
~26,500 |
|
CRC |
~152,810 |
|
NSCLC |
~204,850 |
|
HNSCC |
~63,750 |
|
Ovarian cancer |
~19,700 |
The list is indicative and not exhaustive…
Anti- C-C Motif Chemokine Receptor 8 Antibodies Drug Chapters
The drug chapter segment of the anti-CCR8 antibodies Market report encloses a detailed analysis of early and mid-stage anti-CCR8 antibodies. It also helps understand the clinical trial details of anti-CCR8 antibodies, expressive pharmacological action, agreements and collaborations, approval, and patent details, advantages and disadvantages of each included drug, and the latest news and press releases.
Anti-CCR8 Antibodies Emerging Therapies
BMS-986340 (ONO-7427) + OPDIVO: BMS/Ono Pharma
BMS-986340 is an anti-CCR8 IgG1 biologic with an enhanced non-fucosylated (NF) Fc region that binds to CCR8 and effectively depletes regulatory T cells while sparing effector CD8 T cells. The drug is currently being investigated in a Phase I/II (NCT04895709) clinical trial for solid tumors in the US, EU, and Japan.
In its April 2025 annual presentation, the company reported that ONO-7427, an anti-CCR8 antibody, is undergoing clinical evaluation in trial NCT04895709 for solid tumors, with the study being conducted in Japan, the US, and the EU. The trial’s primary completion is anticipated within fiscal year 2025.
GS-1811 ± Zimberelimab: Gilead Sciences
GS-1811, a potentially first-in-class immunotherapy, is designed to selectively deplete immunosuppressive tumor-infiltrating T regulatory cells in the tumor microenvironment and is currently in Phase I (NCT05007782) clinical development as a possible treatment for patients with solid tumors.
In December 2023, Gilead Sciences and Jounce Therapeutics amended their existing license agreement for GS-1811 (formerly JTX-1811), enabling Gilead to buy out remaining contingent payments potentially due under the license agreement executed in August 2020. As part of the transaction, certain operational obligations of the parties related to GS-1811, an anti-CCR8 antibody, set forth in the license agreement have also been terminated. Gilead will acquire certain related intellectual property, including all outstanding rights of Jounce to GS-1811, pursuant to the transaction agreement.
CHS-114 ± LOQTORZI: Coherus Biosciences and Vaccinex
CHS-114, is an investigational IgG1 antibody targeting CCR8, a chemokine receptor highly expressed on Treg cells” in the tumor microenvironment. CHS-114 is designed as a cytolytic antibody to cause depletion of intra-tumoral Treg cells, important regulators of immune suppression and tolerance, through ADCC, or ADCP or both. CHS-114 has shown anti-tumor activity as monotherapy or in combination with anti-PD-1 antibodies in preclinical models.
The Company is enrolling patients with advanced solid tumors and HNSCC in the US in a clinical trial evaluating safety and pharmacokinetics of CHS-114 with and without LOQTORZI (toripalimab) (NCT05635643). The Company is currently evaluating CHS-114 in combination with toripalimab in a Phase Ib clinical study in second-line HNSCC (NCT05635643). The Company also has an ongoing Phase Ib clinical study of CHS114 in combination with LOQTORZI and/or other treatments in participants with advanced solid tumors with the first cohort evaluating gastric cancer (NCT06657144).
In April 2025, Coherus BioSciences announced that the data from its ongoing Phase I clinical trial evaluating CHS-114 in patients with recurrent/metastatic HNSCC was presented at the 2025 American Association for Cancer Research (AACR) Annual Meeting, taking place April 25-30, 2025.
Comparison of Key Emerging Anti- C-C Motif Chemokine Receptor 8 (CCR8) Antibodies | ||||
|
Product |
Company |
RoA |
Phase |
Indication |
|
BMS-986340 (ONO-7427) + OPDIVO |
BMS/Ono Pharma |
IV |
I/II |
Advanced solid tumors |
|
CHS-114 ± LOQTORZI |
Coherus Biosciences and Vaccinex |
IV |
I |
Solid Tumors
|
|
GS-1811 ± Zimberelimab |
Gilead Sciences |
IV |
I |
Advanced solid tumors |
|
BAY3375968 ± Pembrolizumab |
Bayer |
IV infusion |
I |
Advanced solid tumors |
|
BGB-A3055 ± Tislelizumab/ Chemotherapy |
BeOne Medicines, formerly BeiGene |
IV |
I |
Advanced or metastatic solid tumors |
Note: Detailed emerging therapies assessment will be provided in the final report...
Anti-CCR8 Antibodies Market Outlook
The anti- CCR8 inhibitor market is expected to witness substantial growth in the coming years, driven by the increasing prevalence of autoimmune diseases, robust clinical pipeline activity, and expanding regulatory approvals.
In recent years, there has been increasing interest in the development of CCR8-targeted therapies for the treatment of solid tumors. Several promising candidates are currently under investigation, including BMS-986340 (ONO-7427) in combination with OPDIVO by Bristol Myers Squibb and Ono Pharma, CHS-114 in combination with LOQTORZI by Coherus Biosciences and Vaccinex—targeting various solid tumors including head and neck cancer and gastric cancer—and GS-1811 combined with Zimberelimab by Gilead Sciences for advanced solid tumors. Additional investigational therapies include BAY3375968 with Pembrolizumab from Bayer and BGB-A3055 alongside Tislelizumab or chemotherapy from BeOne Medicines, formerly BeiGene, both focused on advanced or metastatic solid tumors. Parallel to this, there has also been growing exploration of anti-CCR8 antibodies for a wide range of conditions, including several cancer types such as gastric cancer, CRC, TNBC, NSCLC, melanoma, HNSCC, pancreatic adenocarcinoma, and ovarian cancer, as well as autoimmune and inflammatory diseases.
Overall, this is an exciting new class with great potential for development. The maturation of current studies over the next few years will lead to a better understanding of anti-CCR8 antibodies and define their role in autoimmune indications.
Anti-CCR8 Antibodies Market Overview
In recent years, there has been a growing interest in the exploration of anti-CCR8 antibodies for a variety disorders, encompassing cancer indications (gastric cancer, CRC, TNBC, NSCLC, HNSCC, melanoma, ovarian cancer, and others), and others, autoimmune and inflammatory diseases, and other indications.
Further details related to country-based variations are provided in the report…
Anti- C-C Motif Chemokine Receptor 8 Antibodies Drugs Uptake
This section focuses on the uptake rate of potential approved and emerging anti-CCR8 antibodies expected to be launched in the market during 2025–2040.
Anti- C-C Motif Chemokine Receptor 8 Antibodies Pipeline Development Activities
The Anti-CCR8 Antibodies pipeline report provides insights into different Anti-CCR8 Antibodies clinical trials within Phase I/II and Phase I. It also analyzes key Anti-CCR8 Antibodies companies involved in developing targeted therapeutics.
The presence of numerous drugs under different stages is expected to generate immense opportunity for anti-CCR8 antibodies market growth over the forecast period.
Anti-CCR8 Antibodies Pipeline Development Activities
The Anti-CCR8 Antibodies clinical trials analysis report covers information on collaborations, acquisitions and mergers, licensing, and patent details for anti-CCR8 antibodies therapies.
Latest KOL Views on Anti-CCR8 Antibodies
To keep up with current and future Anti-CCR8 Antibodies market trends, we take Industry Experts’ opinions working in the domain through primary research to fill the data gaps and validate our secondary research. Industry experts were contacted for insights on anti-CCR8 antibodies' evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, drug uptake, along challenges related to accessibility.
DelveInsight’s analysts connected with 10+ KOLs to gather insights; however, interviews were conducted with 5+ KOLs in the 7MM. Centers such as Duke University School of Medicine, and others.
Their opinion helps understand and validate current and emerging therapy treatment patterns or anti-CCR8 antibodies’ market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the Anti-CCR8 Antibodies market and the unmet needs.
|
KOL Views |
|
“CCR8 is an attractive target because it allows us to deplete the most suppressive Tregs in the tumor without affecting the broader immune system—something we’ve struggled with in past approaches. By focusing on CCR8+ Tregs, we’re aiming to avoid the immune-related toxicities that come with systemic Treg depletion.” Researcher, Duke University School of Medicine, US |
Anti-CCR8 Antibodies Market Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving treatment landscape.
Anti-CCR8 Antibodies Market Access and Reimbursement
Reimbursement may be referred to as the negotiation of a price between a manufacturer and payer that allows the manufacturer access to the market. It is provided to reduce the high costs and make the essential drugs affordable. Health Technology Assessment (HTA) plays an important role in reimbursement decision-making and recommending the use of a drug. These recommendations vary widely throughout the seven major markets, even for the same drug.
In the US healthcare system, both Public and Private health insurance coverage are included. Also, Medicare and Medicaid are the largest government-funded programs in the US. The major healthcare programs including Medicare, Continuing Medical Education (CME) program, the Children's Health Insurance Program (CHIP), and the state and federal health insurance marketplaces are overseen by the Centers for Medicare & Medicaid Services (CMS). Other than these, Pharmacy Benefit Managers (PBMs), and third-party organizations that provide services, and educational programs to aid patients are also present.
The Anti-CCR8 Antibodies market report further provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of approved therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
The abstract list is not exhaustive, will be provided in the final report....
Scope of the Anti-CCR8 Antibodies Market Report
- The Anti-CCR8 Antibodies market report covers a segment of key events, an executive summary, and a descriptive overview of the anti-CCR8 antibodies, explaining its mechanism, and therapies.
- Comprehensive insight into the anti-CCR8 antibodies competitive landscape, and forecasts, the future growth potential of treatment rate, drug uptake, and drug information have been provided.
- Additionally, an all-inclusive account of the emerging therapies and the elaborative profiles of late-stage and prominent therapies will impact the current landscape.
- A detailed review of the anti-CCR8 antibodies market, historical and forecasted market size, market share by therapies, detailed assumptions, and rationale behind our approach is included in the Anti-CCR8 Antibodies market report, covering the 7MM drug outreach.
- The Anti-CCR8 Antibodies market report provides an edge while developing business strategies, by understanding trends, through SWOT analysis, expert insights/KOL views, and treatment preferences that help shape and drive the 7MM anti-CCR8 antibodies market.
Anti- C-C Motif Chemokine Receptor 8 (CCR8) Antibodies Market Report Insights
- Anti-CCR8 antibodies Targeted Patient Pool
- Anti-CCR8 AntibodiesTherapeutic Approaches
- Anti-CCR8 antibodies Pipeline Analysis
- Anti-CCR8 antibodies Market Size
- Anti-CCR8 Antibodies Market Trends
- Existing and future Market Opportunities
Anti-CCR8 Antibodies Market Report Key Strengths
- 16 years Forecast
- The 7MM Coverage
- Key Cross Competition
- Anti-CCR8 AntibodiesDrugs Uptake
- Key Anti-CCR8 Antibodies Market Forecast Assumptions
Anti-CCR8 Antibodies Market Report Assessment
- Current Anti-CCR8 Antibodies Treatment Practices
- Anti-CCR8 Antibodies Unmet Needs
- Anti-CCR8 AntibodiesPipeline Product Profiles
- Anti-CCR8 AntibodiesMarket Attractiveness
- Qualitative Analysis (SWOT and Conjoint)
- Anti-CCR8 Antibodies Market Drivers
- Anti-CCR8 Antibodies Market Barriers
Key Questions Answered In The Anti-CCR8 Antibodies Market Report:
- What was the total anti-CCR8 antibodies market size, the market size by therapies, market share (%) distribution, and what would it look like in 2040? What are the contributing factors for this growth?
- Which Anti-CCR8 Antibodies drug is going to be the largest contributor in 2040?
- Which is the most lucrative anti-CCR8 antibodies market?
- What are the risks, burdens, and unmet needs of treatment with anti-CCR8 antibodies? What will be the growth opportunities across the 7MM for the patient population of anti-CCR8 antibodies?
- What are the key factors hampering the growth of the Anti-CCR8 Antibodies market?
- What are the indications for which recent novel therapies and technologies have been developed to overcome the limitations of existing treatments?
- What key designations have been granted to the therapies for anti-CCR8 antibodies?
- What is the cost burden of approved Anti-CCR8 Antibodies therapies on the patient?
- Patient acceptability in terms of preferred therapy options as per real-world scenarios?
- What are the country-specific accessibility issues of expensive, recently approved Anti-CCR8 Antibodies therapies?
Reasons to buy Anti-CCR8 Antibodies Market Forecast Report
- The Anti-CCR8 Antibodies market report will help develop business strategies by understanding the latest trends and changing dynamics driving the anti-CCR8 antibodies market.
- Understand the existing Anti-CCR8 Antibodies market opportunities in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain) the United Kingdom, and Japan.
- Identifying strong upcoming Anti-CCR8 Antibodies companies in the Anti-CCR8 Antibodies market will help devise strategies to help get ahead of competitors.
- Detailed analysis and ranking of indication-wise emerging therapies under the conjoint analysis section to provide visibility around leading indications.
- Highlights of access and reimbursement policies of approved Anti-CCR8 Antibodies therapies, barriers to accessibility of expensive off-label therapies, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing Anti-CCR8 Antibodies market so that the upcoming Anti-CCR8 Antibodies companies can strengthen their development and launch strategy