Her2+ Non Small Cell Lung Cancer Market
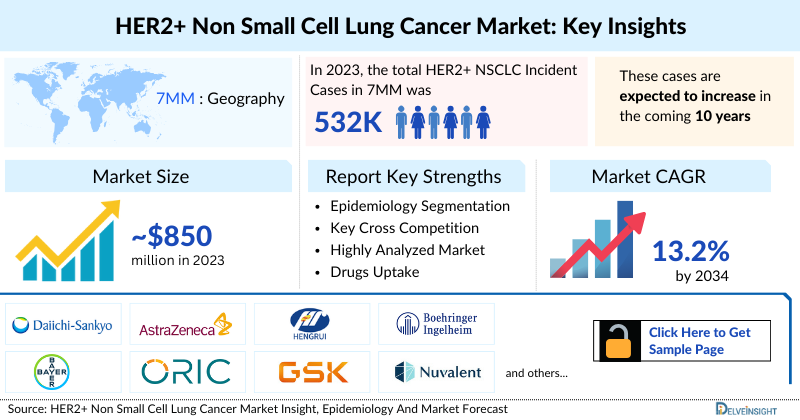
- The HER2+ NSCLC Market (Mutant, Overexpression, and Amplification) in the 7MM was USD ~850 million in 2023 and is expected to increase to USD ~3,110 million by 2034, at a CAGR of 13.2%.
- NSCLC comprises approximately 80– 85% of all lung cancer diagnoses, with HER2+ gene mutation found in approximately 1–4% of NSCLC cases. Additionally, HER2+ amplification and overexpression are observed in about 2–5% and 2–30% of NSCLC cases, respectively, in the US.
- Currently, the regimen of HER2-mutant NSCLC treatment involves the use of Chemotherapy, Targeted therapy, Immunotherapy, Laser therapy, Photodynamic therapy, Radiation therapy, Surgery, and some other options.
- ENHERTU (trastuzumab deruxtecan) stands as the only FDA approved therapy for the treatment of adult patients with unresectable or metastatic NSCLC whose tumors have activating HER2+ (ERBB2) mutations and who have received prior systemic therapy. The drug received accelerated approval in August 2022, however, its approval is limited to later-line settings, leaving significant unmet needs in first-line treatment.
- Recently, novel, more selective, oral HER2+ TKIs have been developed to improve outcomes in NSCLC with HER2+ mutations, looking to challenge ENHERTU’S dominance
- Potential drugs in the HER2+ NSCLC Pipeline include Pyrotinib (Jiangsu HengRui Medicine), Zongertinib (Boehringer Ingelheim), BAY 2927088 (Bayer), FWD1509 (Forward Pharmaceuticals), ORIC-114 (ORIC Pharmaceuticals), IAM1363 (Iambic Therapeutics), Firmonertinib (ArriVent BioPharma), XMT-2056 (Mersana Therapeutics/ GSK), and others in different stages of clinical development. The launch of these therapies would significantly impact the HER2+ NSCLC market during the forecast period (2024-2034).
- Boehringer's zongertinib and Bayer's BAY2927088 are neck and neck in a race to become the first targeted small molecule approved for HER2+-mutated NSCLC.
- The HER2+ NSCLC Market Dynamics are anticipated to change in the coming years owing to the improvement in the diagnosis methodologies, raising awareness of the disease, and incremental healthcare spending across the world. However, the poor prognosis, late diagnosis, and lack of effective biomarkers of HER2+ NSCLC, particularly with brain metastases, underscores a significant unmet need for more effective treatments.
Request for Unlocking the Sample Page of the "HER2+ NSCLC Treatment Market"
Key Factors Driving HER2+ Non Small Cell Lung Cancer Market
HER2+ Non Small Cell Lung Cancer Rising Prevalence
HER2+ mutations occur in approximately 1–4% of NSCLC cases, with amplification and overexpression seen in 2–5% and 2–30% of cases respectively in the US. NSCLC accounts for roughly 80–85% of all lung cancer diagnoses, and the growing identification of HER2+ subtypes is increasing the need for targeted therapies.
HER2+ Non Small Cell Lung Cancer Current Treatment Landscape
Treatment options for HER2+ NSCLC include chemotherapy, targeted therapy, immunotherapy, surgery, radiation, laser therapy, and photodynamic therapy. ENHERTU (trastuzumab deruxtecan) is currently the only FDA-approved therapy for unresectable or metastatic HER2+ NSCLC in later-line settings, leaving room for expansion in earlier lines of treatment.
HER2+ Non Small Cell Lung Cancer Market Drivers
The HER2+ NSCLC market is expected to expand due to improved diagnostic approaches, increased awareness of HER2 mutations, and rising healthcare investments. Novel oral HER2 tyrosine kinase inhibitors (TKIs) are in development, aiming to improve patient outcomes and broaden treatment options.
HER2+ Non Small Cell Lung Cancer Clinical Trials and Competitive Landscape
The pipeline features multiple candidates in different stages of development, including Pyrotinib (Jiangsu HengRui Medicine), Zongertinib (Boehringer Ingelheim), BAY 2927088 (Bayer), FWD1509 (Forward Pharmaceuticals), ORIC-114 (ORIC Pharmaceuticals), IAM1363 (Iambic Therapeutics), Firmonertinib (ArriVent BioPharma), and XMT-2056 (Mersana Therapeutics/GSK). Zongertinib and BAY2927088 are leading in clinical trials as potential first targeted small-molecule therapies for HER2+-mutated NSCLC, reflecting a highly competitive landscape.
DelveInsight’s "HER2+ NSCLC Market Insight, Epidemiology, and Market Forecast – 2034" report delivers an in-depth understanding of HER2+ NSCLC, historical and forecasted epidemiology as well as the HER2+ NSCLC market trends in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
The HER2+ NSCLC Treatment Market report provides current treatment practices, emerging drugs, HER2+ NSCLC market share of individual therapies, and current and forecasted HER2+ NSCLC market size from 2020 to 2034, segmented by seven major markets. The report also covers current HER2+ NSCLC treatment practices/algorithms and unmet medical needs to curate the best of the opportunities and assess the underlying potential of the market.
HER2+ NSCLC Treatment Market: Understanding and Algorithm
HER2+ also known as ERBB2, is a receptor tyrosine kinase encoded by the ERBB2 proto-oncogene localized on the long arm of chromosome 17 (17q21). It is a member of the EGFR/ErbB family, which comprises HER1/EGFR, HER2+, HER3 and HER4. Unlike the other members of the EGFR/ErbB family, no ligand is known for HER2+. Its activation through homodimerization or heterodimerization with another ligand-bound HER family member leads to its cross-phosphorylation and activation of the tyrosine kinase catalytic domain.
The three main HER2+ dysregulation mechanisms have been described in NSCLCs: gene mutation, gene amplification, and protein overexpression. However, these different types of alterations seem to originate from different mechanisms and induce different biological and clinical consequences. The impact of these HER2+ alterations on NSCLC tumor cells is on the biology and behavior of patients and also plays significance in therapeutic strategies for NSCLC patients. The symptoms of HER2+ NSCLC may include a persistent cough, chest pain, and shortness of breath, wheezing, loss of appetite, weight loss, and unusual tiredness.
HER2+ NSCLC Diagnosis
The diagnosis and staging of NSCLC are often done at the same time. The tests and procedures used in the diagnosis of NSCLC includes:
- Physical exam and history
- Laboratory tests
- Chest X-ray
- CT scan (CAT scan)
- Sputum cytology
- Thoracentesis
- Biopsy
- Fine-needle Aspiration (FNA) biopsy of the lung
- Bronchoscopy
- Thoracoscopy
- Lymph node biopsy
The specific diagnosis of HER2+ NSCLC, which is used to confirm the mutation type, is as follows:
- FISH for HER2+ amplifications
- Immunohistochemical staining for HER2+ overexpression
- Next-generation Sequencing (NGS) for mutation
Further details related to diagnosis will be provided in the report…
HER2+ NSCLC Treatment
The results of the standard treatment of HER2+ NSCLC are poor except for the most localized cancers. The newly diagnosed patients with HER2+ NSCLC are potential candidates for studies evaluating new forms of treatment. There are different types of treatment available for HER2+ NSCLC; however, mainly different types of standard treatment are used, which include surgery, radiation therapy, chemotherapy, targeted therapy, immunotherapy, laser therapy, photodynamic therapy, cryosurgery, electrocautery, watchful waiting.
ENHERTU is the only FDA approved drug used as the treatment approach for patients with HER2+ NSCLC. It received FDA approval in August 2022 in the United States for the treatment of HER2+-mutant NSCLC patients who have undergone prior systemic therapy. This approval has significantly transformed therapeutic approaches for pretreated HER2+-mutant NSCLC.
Further details related to treatment will be provided in the report…
HER2+ Non-small Cell Lung Cancer Epidemiology
The HER2+ NSCLC epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by the total incident cases of NSCLC, gender-specific cases of NSCLC, age-specific cases of NSCLC, total incident cases of NSCLC by histology, total incident cases of NSCLC by stage, and total incident cases of advanced HER2+ NSCLC (mutation, overexpression and amplification) in the 7MM covering the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan from 2020 to 2034.
- In 2023, the total HER2+ NSCLC Incident Cases in 7MM was ~531,800, out of which the contribution of the US was ~38%.
- Among EU4 and the UK, Germany accounted for the highest number of HER2+ NSCLC cases whereas Spain accounted for the lowest number of cases in 2023.
- In the US total diagnosed cases of NSCLC by stage were observed to be ~59,400, ~16,900, ~26,700, ~11,200, and ~88,300 cases of stage I, stage II, stage IIIA, stage IIIB, and stage IV, respectively, in 2023.
- In 2023, total incident cases of advanced HER2+ NSCLC according to HER2+ gene mutation, HER2+ gene overexpression, and HER2+ gene amplification were ~6,000, ~32,400, and ~8,100 respectively in the US.
HER2+ NSCLC Drug Chapters
HER2+ NSCLC Marketed Drugs
- ENHERTU (trastuzumab deruxtecan): Daiichi Sankyo and AstraZeneca
ENHERTU (trastuzumab deruxtecan) is a HER2+-directed antibody and topoisomerase inhibitor conjugate indicated for the treatment of adult patients with unresectable or metastatic NSCLC whose tumors have activating HER2+ (ERBB2) mutations, as detected by an FDA-approved test, and who have received prior systemic therapy.
The drug received approval by the US FDA in August 2022 for treating adult patients with previously treated HER2+-mutant metastatic NSCLC. This indication is approved under accelerated approval based on objective response rate (ORR) and duration of response (DoR). Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial. In August 2023, ENHERTU also secured approval from Japan’s MHLW for HER2+ NSCLC, followed by approval in Europe the following month. The company is now focused on expanding ENHERTU’s use in the first-line setting through a Phase III clinical trial, with topline data expected by 2025, building on its success in the second-line setting.
To be continued in the report….
HER2+ NSCLC Emerging Drugs
- Zongertinib (BI-1810631): Boehringer Ingelheim
Zongertinib (also known as BI 1810631) is an investigational oral HER2+-specific TKI that is being developed as a potential treatment for HER2+-mutated NSCLC. The drug was granted FDA Fast Track Designation in 2023; then, in 2024, it was granted Breakthrough Therapy Designation by the US FDA and China CDE for the treatment of adult patients with advanced NSCLC whose tumors have activating HER2+ mutations and who have received a prior systemic therapy.
Currently, the company is evaluating the drug in a Phase III (Beamion LUNG-2) clinical trial for first-line treatment in patients with unresectable, locally advanced, or metastatic Non-squamous NSCLC harboring HER2+ tyrosine kinase domain mutations. In addition, the Phase I Beamion-LUNG-1-trial is investigating zongertinib as a monotherapy in people with NSCLC whose cancer harbors HER2+ mutations. In September 2024, the company reported positive results from a Phase Ib primary analysis of Cohort 1 of the Beamion LUNG-1 trial.
- BAY 2927088: Bayer
BAY 2927088 is an oral, small-molecule tyrosine kinase inhibitor that potently inhibits mutant HER2+, including HER2+ exon 20 insertions and HER2+ point mutations, as well as EGFR, with high selectivity for mutant vs wild-type EGFR. The drug received Breakthrough Therapy designation, in February 2024, for the treatment of adult patients with unresectable or metastatic NSCLC whose tumors have activating HER2+ (ERBB2) mutations and who have received prior systemic therapy.
Currently, the drug is being evaluated in Phase III (SOHO-02) clinical trial for first-line therapy in patients with locally advanced or metastatic NSCLC With HER2+-activating mutations. According to the Q2 2024 presentation, the company anticipates the submission of BAY 2927088 for second-line HER2+-mutated NSCLC in 2025.
HER2+ NSCLC Drugs Market Insights
- Tyrosine kinase inhibitors (TKIs)
TKIs are a class of targeted therapies designed to block the activity of tyrosine kinases, which are enzymes that play a critical role in the signaling pathways that regulate cell growth, division, and survival. There is a lack of approved TKIs in HER2+-mutant NSCLC. Even though HER2+-targeted therapies have a well-established role in breast cancer care, their use in lung cancer is now generating some excitement. Potential emerging TKIs for HER2-mutant NSCLC Pipeline includes Zongertinib (Boehringer Ingelheim), BAY 2927088 (Bayer), IAM1363 (Iambic Therapeutics), Firmonertinib (ArriVent BioPharma), NVL-330 (Nuvalent). Emerging TKI's have shown signs of activity in patients with brain metastases.
- Antibody drug conjugate (ADC)
Currently, only one ADC is approved for the treatment of HER2+ NSCLC. The ADC that has made the most waves in the HER2+-mutant NSCLC space is Daiichi Sankyo and AstraZeneca’s ENHERTU. It is important to note that ADCs are considered a dressed-up form of chemotherapy. Therefore, some side effects are expected. Particular attention should be given to side effects, especially for patients with lung cancer, such as ILD (interstitial lung disease) or pneumonitis.
Further detailed analysis will be provided in the report….
HER2+ NSCLC Market Outlook
HER2+ alterations represent important oncogenic drivers in NSCLC. During the past couple of years, progress has been made toward defining HER2+-driven disease and determining the benefit of different classes of agents targeting HER2+. Currently, platinum-based chemotherapy with/without immunotherapy is the preferred first-line treatment in patients with advanced or metastatic HER2+ NSCLC. The longtime standard of care in the space, chemotherapy, immunotherapy, Checkpoint inhibitors, and targeted cell therapy has produced a median Progression-free Survival (PFS) of only approximately 4 months historically, underscoring the need for more effective treatment options in the space.
ENHERTU (T-DXd) received FDA approval in August 2022 in the United States for the treatment of HER2+-mutant NSCLC patients who have undergone prior systemic therapy. This approval has significantly transformed therapeutic approaches for pretreated HER2+-mutant NSCLC. In August 2023, ENHERTU also secured approval from Japan’s MHLW for HER2+ NSCLC, followed by approval in Europe the following month.
The landscape for treating HER2+ NSCLC is evolving rapidly as research continues to uncover the genetic underpinnings of these tumors. Emerging drugs like Pyrotinib (Jiangsu HengRui Medicine), Zongertinib (Boehringer Ingelheim), and BAY2927088 (Bayer) being developed for HER2+-mutant NSCLC are gaining significant attention. Development of these novel HER2+ TKIs, such as represents a shift toward more selective and effective targeted treatments. In addition to these, the emerging pipeline features candidates such as FWD1509 (Forward Pharmaceuticals), IAM1363 (Iambic Therapeutics), ORIC-114 (ORIC Pharmaceuticals), Firmonertinib (ArriVent BioPharma), and others are being evaluated in different stages of clinical development, contributing to increased innovation and competition in the HER2+ NSCLC space. Furthermore, combination therapies are being investigated to enhance the efficacy of anti-HER2+ agents.
- Among the 7MM, the US accounted for the largest market size of HER2+ NSCLC i.e., USD ~540 million in 2023.
- Among EU4 and the UK, Germany captured the maximum market size in 2023, followed by France in the same year.
- In 2023, among the therapies for HER2+ NSCLC, the highest revenue was generated by Checkpoint inhibitors ± Chemotherapy, i.e., USD ~480 million in the US.
Recent Developments in the HER2+ NSCLC Treatment Market Landscape
- In September 2024, AstraZeneca presented the first data of the ENHERTU monotherapy cohort for second or later-line treatment at WCLC from the Phase Ib clinical trial for HER2+ positive nonsquamous NSCLC (DESTINY-Lung03), building on data from the DESTINY-Lung01 Phase II trial.
- In September 2024, Boehringer Ingelheim reports positive results from a Phase Ib primary analysis of Cohort 1 of the Beamion LUNG-1 trial evaluating zongertinib in pretreated patients with advanced NSCLC with activating HER2+ mutations.
- In August 2024, Bayer announced that the first patient had been enrolled in the global Phase III SOHO-02 trial, an open-label, randomized, multicenter clinical trial, assessing the efficacy and safety of investigational agent BAY 2927088 as first-line therapy in patients with advanced NSCLC, whose tumors have activating HER2+ mutations.
- In June 2024, Bayer presented the safety and clinical activity of BAY 2927088 from a Phase I/II trial expansion cohort in patients with HER2+-mutant NSCLC at the 2024 ASCO annual meeting.
To be continued in the report….
HER2+ NSCLC Drugs Uptake
This section focuses on the uptake rate of potential drugs expected to be launched in the market during 2020–2034. The landscape of HER2+ NSCLC treatment has experienced an uptake of novel drugs. These innovative therapies are redefining standards of care. Furthermore, the increased uptake of these transformative drugs is a testament to the unwavering dedication of physicians, oncology professionals, and the entire healthcare community in their tireless pursuit of advancing treatment care. This momentous shift in treatment paradigms is a testament to the power of research, collaboration, and human resilience.
Boehringer's zongertinib and Bayer's BAY2927088 (both are oral agents) have had differing development histories, but they are now neck and neck in a race to become the first targeted small molecule approved for HER2+-mutated NSCLC. Experts agrees that zongertinib has better tolerability than BAY 2927088. It is important to highlight that we do not see much of future for pyrotinib since zongertinib and BAY2927088 have shown nearly 70% ORR in the Phase I/II trials.
Further detailed analysis of emerging therapies drug uptake in the report…
HER2+ NSCLC Pipeline Development Activities
The HER2+ NSCLC therapeutics market report provides insights into different therapeutic candidates in Phase III, Phase II, and Phase I stage. It also analyzes key HER2+ NSCLC Companies involved in developing targeted therapeutics.
Pipeline Development Activities
The HER2+ NSCLC therapeutics market report covers detailed information on collaborations, acquisitions and mergers, licensing, and patent details for HER2+ NSCLC emerging therapies.
KOL- Views
To keep up with current market trends, we take KOLs and SMEs' opinions working in the domain through primary research to fill the data gaps and validate our secondary research. Some of the leaders like MD, Professor and Vice Chair Department of Critical Care Medicine and Director, PhD, and others. Their opinion helps to understand and validate current and emerging therapies and treatment patterns or HER2+ NSCLC Market Trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the HER2+ NSCLC unmet needs.
Delveinsight’s analysts connected with 20+ KOLs to gather insights; however, interviews were conducted with 10+ KOLs in the 7MM. Versailles Saint Quentin University, France; Fred Hutchinson Cancer Center, US; Lung Clinic Grosshansdorf, Germany; Sylvester Comprehensive Cancer Center, US; University Hospital Münster, Münster, Germany; Johns Hopkins University School of Medicine, US; Massachusetts General Hospital, US; Nagasaki University, Japan; Vall d’Hebron University Hospital, Spain; Georgetown University, US, etc., were contacted. Their opinion helps understand and validate HER2+ NSCLC epidemiology and market trends.
HER2+ NSCLC Therapeutics Market: Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT and conjoint analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the Analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving HER2+ NSCLC Treatment Market Landscape.
The analyst analyzes multiple emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. In efficacy, the trial’s primary and secondary outcome measures are evaluated. Further, the therapies’ safety is evaluated wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials.
HER2+ NSCLC Therapeutics Market Access and Reimbursement
ENHERTU: Patient Savings Program
Eligible patients may pay as little as USD 0 per ENHERTU prescription. The annual benefit can be used for the cost of the drug itself and may also cover up to USD 100 in infusion costs per administration. There are no income requirements to participate in the program. Patients who are residents of Massachusetts or Rhode Island are not eligible for infusion assistance.
Key eligibility criteria:
- Patients are eligible for the program if they meet all of the following criteria:
- The patient may be eligible for this offer if they are insured by commercial insurance and their insurance does not cover the full cost of their prescription.
Patients who are enrolled in a state or federally funded prescription insurance program are not eligible for this offer. This includes patients enrolled in Medicare Part B, Medicare Part D, Medicaid, Medigap, Veterans Affairs (VA), Department of Defense (DoD) programs or TRICARE, and patients who are Medicare-eligible and enrolled in an employer-sponsored group waiver health plan or government-subsidized prescription drug benefit program for retirees. Suppose the patient is enrolled in a state or federally funded prescription insurance program. In that case, they may not use this program even if they elect to be processed as an uninsured (cash-paying) patient. This offer is not insurance and is restricted to residents of the United States and Puerto Rico.
Further detailed analysis will be provided in the report…
HER2+ NSCLC Treatment Market Report Scope
- The HER2+ NSCLC therapeutics market report covers a descriptive overview, explaining its causes, signs and symptoms, pathogenesis, and currently available therapies.
- Comprehensive insight has been provided into HER2+ NSCLC epidemiology and treatment.
- Additionally, an all-inclusive account of both the current and emerging therapies for HER2+ NSCLC is provided, along with the assessment of new therapies, which will have an impact on the current treatment landscape.
- A detailed review of the HER2+ NSCLC therapeutics market; historical and forecasted is included in the report, covering the 7MM drug outreach.
- The HER2+ NSCLC therapeutics market report provides an edge while developing business strategies, by understanding trends shaping and driving the 7MM HER2+ NSCLC market.
HER2+ NSCLC Treatment Market Report Insights
- Patient-based HER2+ NSCLC Market Forecasting
- Therapeutic Approaches
- HER2+ NSCLC Pipeline Drugs Analysis
- HER2+ NSCLC Market Size and Trends
- HER2+ NSCLC Drugs Market Opportunities
- Impact of Upcoming Therapies
HER2+ NSCLC Treatment Market Report Key Strengths
- 11 Years HER2+ NSCLC Market Forecast
- 7MM Coverage
- HER2+ NSCLC Epidemiology Segmentation
- Key Cross Competition
- Highly Analyzed HER2+ NSCLC Drugs Market
- HER2+ NSCLC Drugs Uptake
HER2+ NSCLC Treatment Market Report Assessment
- Current HER2+ NSCLC Treatment Market Practices
- HER2+ NSCLC Unmet Needs
- HER2+ NSCLC Pipeline Drugs Profiles
- HER2+ NSCLC Drugs Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
FAQs
- What was the HER2+ NSCLC drugs market share (%) distribution in 2020 and what it would look like in 2034?
- What would be the HER2+ NSCLC treatment market size as well as market size by therapies across the 7MM during the study period (2020–2034)?
- Which country will have the largest HER2+ NSCLC market size during the study period (2020–2034)?
- What are the disease risks, burdens, and unmet needs of HER2+ NSCLC?
- What is the historical HER2+ NSCLC patient pool in the United States, EU4 (Germany, France, Italy, and Spain), and the UK, and Japan?
- What is the distribution of HER2+ mutation, overexpression, and amplification in NSCLC?
- What will be the growth opportunities across the 7MM concerning the patient population of HER2+ NSCLC?
- How many emerging therapies are in the mid-stage and late stage of development for the HER2+ NSCLC treatment?
- What are the key collaborations (Industry–Industry, Industry-Academia), Mergers and acquisitions, and licensing activities related to HER2+ NSCLC therapies?
- What are the recent novel therapies, targets, HER2+ NSCLC mechanisms of action, and technologies developed to overcome the limitations of existing therapies?
- What are the clinical studies going on for HER2+ NSCLC and their status?
- What are the key designations that have been granted for the HER2+ NSCLC emerging therapies?
Reasons to Buy
- The HER2+ NSCLC therapeutics market report will help in developing business strategies by understanding trends shaping and driving HER2+ NSCLC drugs market.
- To understand the future market competition in the HER2+ NSCLC drugs market and Insightful review of the SWOT analysis of HER2+ NSCLC.
- Organize sales and marketing efforts by identifying the best opportunities for HER2+ NSCLC in the US, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
- Identification of strong upcoming players in the HER2+ NSCLC drugs market will help in devising strategies that will help in getting ahead of competitors.
- Organize sales and marketing efforts by identifying the best opportunities for the HER2+ NSCLC drugs market.
- To understand the future market competition in the HER2+ NSCLC drugs market.
Stay Updated with us for Recent Articles
- The Next Chapter in NSCLC Treatment Space: Recent Discoveries and Innovations
- Novel mutation-targeting therapies in the horizon to relieve the global healthcare burden NSCLC poses
- Non-Small Cell Lung Cancer Market: Treatments and Market Forecast
- Evaluating Key Advancements and Emerging Therapies in EGFR-Non Small Cell Lung Cancer Treatment Market
- Novel Insights Into The Non-Small Cell Lung Cancer Market
- Evolving Landscape for Rare Biomarkers in Non-Small Cell Lung Cancer
- Latest DelveInsight Blogs
Related Infographics of the Report
- BRAF Non-Small Cell Lung Cancer (BRAF+ NSCLC) Market
- ALK Non-Small Cell Lung Cancer (ALK+ NSCLC) Market
- c-MET Non-Small Cell Lung Cancer (c-MET+ NSCLC) Market
- EGFR Non-Small Cell Lung Cancer (EGFR+ NSCLC) Market
- PD-L1Non-Small Cell Lung Cancer (PD-L1+ NSCLC) Market
Stay Updated with us for Recent Articles @ Latest DelveInsight Blogs