Novel Insights Into The Non-Small Cell Lung Cancer Market
Nov 29, 2024
Table of Contents
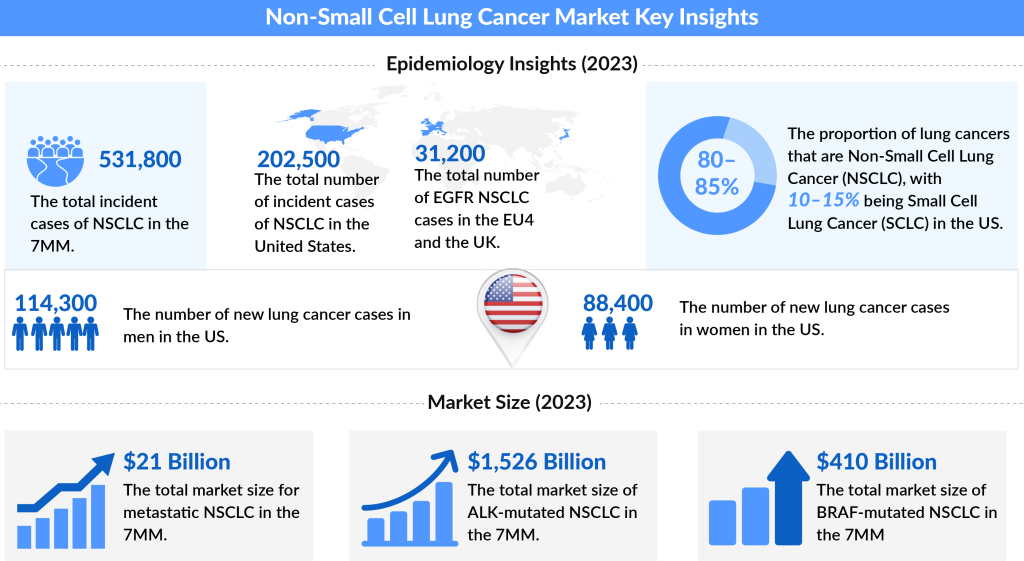
Non-small cell lung cancer (NSCLC) is the most common cancer, accounting for approximately 85% of all lung cancers. the total number of incident cases of NSCLC across the 7MM was approximately 500,000 in 2022 and is projected to reach around 600,000 cases by 2032.
Studies show that non-small cell lung cancer affects people aged above 65 years. A few people diagnosed with non-small cell lung cancer lie in the age group of 40 to 45 years.
Downloads
Click Here To Get the Article in PDF
Recent Articles
As per DelveInsight’s assessment, every year, about 7 out of 10 patients in the United States are diagnosed with metastatic NSCLC Stages IIIB and IV.
Non-small cell lung cancer is mainly subcategorized into adenocarcinomas, squamous cell carcinomas, giant cell carcinomas, and several other types that occur less frequently, including adenosquamous carcinomas and sarcomatoid carcinomas. Non-small cell lung cancer can be located in the mid-chest, but it is also found in other parts of the lung. Even though NSCLCs are associated with cigarette smoking, adenocarcinomas may also be found in patients who have never smoked.
According to the SEER, if the cancer is found only in the part of the body where it started, it is localized (sometimes referred to as Stage I). The stage-specific cases of non-small cell lung cancer are 16%, 22%, 57%, and 4% for the localized, regional, distant, and unknown stages.
Patients of non-small cell lung cancer with resectable disease may be cured by surgery or chemotherapy. Local control can be achieved by radiation therapy in a large number of patients with unresectable diseases. On the other hand, patients with locally advanced unresectable disease may achieve long-term survival with radiation therapy combined with chemotherapy, and patients with advanced metastatic disease may achieve improved survival and palliation of symptoms with chemotherapy, targeted agents, and other supportive measures.
Non-Small Cell Lung Cancer Disease Overview
The approaches for non-small cell lung cancer treatment depend upon the disease stage. There are five stages for NSCLC: stage 0 (zero) and stages I through IV (1 through 4). Different stages have different severity.
Stage 0
Stage 0 of the disease is “in situ” and does not show signs of spreading. This means lung cancer cells are static and haven’t traveled to nearby tissues or cells.
Stage I
Stage I refers to cancer cells that haven’t been spread to any lymph nodes. They are further classified into Stage I and Stage 2 based on their size. Stage IA cancer cells are small and can extend upto 3cm in size. In contrast, Stage IB can range from 3cm to 4cm in size.
Stage II
Stage IIA lung cancer is characterized by tumor size ranging from 4cm to 5cm but does not spread to lymph nodes. However, Stage IIB is 5cm or less but does spread to lymph nodes within the lungs.
Stage III/IV
Stage III tumors are further classified into three subtypes depending upon their size. Stage III tumors are metastatic and spread in the lungs’ lymph nodes. However, they do not spread to nearby spaces and organs. Stage IV tumors spread to more than one area inside the lungs and fluids surrounding the pulmonary cavity, including another bloodstream.
According to the research study of Alaoui et al. (2016), the 5-year survival rates after surgical resection are 60%–80% for Stage I NSCLC and 30%–50% for Stage II non-small cell lung cancer patients. More than 70% of NSCLC patients are diagnosed in advanced stages of metastatic disease. Also, the 5-year survival rate varies between 10% to 15% for Stage IIIA disease and 2% to 5% for Stage IIIA with mediastinal involvement. Stage IIIB non-small cell lung cancer signifies approximately 17.6% of all lung cancers with a 5-year survival rate of 3% to 7%.
Therapies Existing in the Non-Small Cell Lung Cancer Market
Currently, several different types of treatment options are available for non-small cell lung cancer treatment, some of which include Surgery, Radiation therapy, Chemotherapy, Targeted therapy, Immunotherapy, Laser therapy, Photodynamic therapy (PDT), Cryosurgery, and Electrocautery. Presently, some of the FDA-approved drugs for NSCLC treatment include Capmatinib, INC280, Telisotuzumab Vedotin, JNJ-61186372, JNJ-6372, Ensartinib, X-396, Selpercatinib, SAR408701, PADCEV, and many others.
It is observed that, over the past few years, immune checkpoint inhibitors (ICIs) have emerged as one of the major standards of care in treating advanced non-small cell lung cancer (NSCLC) patients either in first line or beyond. To further improve the treatment scenario, several leading pharmaceutical giants, including AstraZeneca, Bristol-Myers-Squibb, AbbVie, Roche, Merck, Novartis, Pfizer, Takeda, Eli Lilly, Immutep, Sanofi, GlaxoSmithKline, and others, are also involved in developing novel therapies for non-small cell lung cancer treatment.
FDA-approved Therapies Available in the Non-Small Cell Lung Cancer Market
The development of immunotherapies and other novel treatments has significantly reshaped the treatment paradigm for NSCLC. Among the most prominent therapies are:
KEYTRUDA (pembrolizumab): Merck
KEYTRUDA, an anti-PD-1 therapy, enhances the immune system’s ability to detect and fight tumors. In January 2023, the FDA approved KEYTRUDA for the treatment of resected stage IB (T2a ≥4 cm), II, or IIIA non-small cell lung cancer (NSCLC) following adjuvant chemotherapy. This marks the fifth indication for KEYTRUDA in NSCLC and makes it the only immunotherapy approved for both adjuvant and metastatic NSCLC, regardless of PD-L1 expression. KEYTRUDA’s broader approval is a significant advantage over TECENTRIQ, which is only approved for PD-L1-positive stage 2–3A NSCLC patients, limiting its use compared to KEYTRUDA’s more inclusive range.
LIBTAYO (cemiplimab-rwlc): Regeneron Pharmaceuticals
LIBTAYO, a fully human PD-1 inhibitor developed using Regeneron’s VelocImmune technology, was approved by the US FDA in November 2022 for first-line treatment of advanced non-small cell lung cancer (NSCLC) in combination with platinum-based chemotherapy. This approval expands its use to patients with no EGFR, ALK, or ROS1 mutations, and it can now be prescribed regardless of PD-L1 levels or tumor histology. LIBTAYO, alongside KEYTRUDA, is one of the few PD-1 inhibitors approved for use with chemotherapy in first-line NSCLC treatment. However, its market share may not necessarily match that of Merck’s KEYTRUDA.
IMFINZI: Medimmune/AstraZeneca
Imfinzi (Durvalumab) is a human monoclonal antibody that binds to PD-L1 and blocks the interaction of PD-L1 with PD-1 and CD80, countering the tumor’s immune-evading tactics and releasing the inhibition of immune responses. This product is given by intravenous infusion and is approved in unresectable, Stage III NSCLC after chemoradiation therapy in the US, Japan, across the EU, and in many other countries, based on the Phase III PACIFIC trial.
OPDIVO: Bristol-Myers Squibb
OPDIVO (Nivolumab) by Bristol-Myers Squibb is a human immunoglobulin (Ig) G4 monoclonal antibody directed against the negative immunoregulatory human cell surface receptor PD-1, with immune checkpoint inhibitory and antineoplastic activities. For NSCLC, it is approved for patients with metastatic NSCLC and progression on or after platinum-based chemotherapy. Patients with EGFR or ALK genomic tumor aberrations should have disease progression on FDA-approved therapy for these aberrations before receiving OPDIVO.
TECENTRIQ: Genentech
TECENTRIQ (Atezolizumab) by Genentech (a subsidiary of Roche) is a PD-L1 blocking antibody. It is an Fc-engineered, humanized, non-glycosylated IgG1 kappa immunoglobulin with a calculated molecular mass of 145 kDa. TECENTRIQ is approved for indications such as NSCLC, SCLC, Urothelial Carcinoma, and Triple-Negative Breast Cancer (TNBC). For NSCLC, it is approved in combination with other drugs, such as bevacizumab, paclitaxel, and carboplatin, for the first-line treatment of adult patients with metastatic non-squamous NSCLC with no EGFR or ALK genomic tumor aberrations, among others.
TAGRISSO: AstraZeneca
TAGRISSO (osimertinib) is a third-generation EGFR-TKI of AstraZeneca. It is a best-in-class, highly selective, irreversible kinase inhibitor of the activating sensitizing EGFR mutation and the resistance mutation T790Mwith clinical activity against CNS metastases. This product is used as first-line treatment of patients with metastatic NSCLC whose tumors have EGFR exon 19 deletions or exon 21 L858R mutations and as a treatment of patients with metastatic EGFRT790M mutation-positive NSCLC, as detected by an FDA-approved test, whose disease has progressed on or after EGFR TKI therapy. In February 2022, it got approved for the adjuvant treatment of patients with early-stage NSCLC.
VIZIMPRO: Pfizer VIZIMPRO
VIZIMPRO (dacomitinib) is oral, a once-daily prescription medicine used to treat NSCLC that has spread to other body parts (metastatic). It is a product of Pfizer VIZIMPRO and is used as a first treatment if your tumor has certain abnormal EGFR genes. Alunbrig (Brigatinib) is a kinase inhibitor used to treat patients with ALK-positive metastatic NSCLC who have progressed on or are intolerant to crizotinib. Accelerated approval was granted for this indication based on tumor response rate and duration of response. ARIAD Pharmaceuticals initially discovered this product, which Takeda later acquired in February 2019.
VITRAKVI: Bayer
VITRAKVI (Larotrectinib) is an oral selective tropomyosin receptor kinase (TRK) inhibitor used for adult treatment. Pediatric patients with solid tumors with a neurotrophic receptor tyrosine kinase (NTRK) gene fusion without a known acquired resistance mutation, are metastatic or where surgical resection is likely to result in severe morbidity, and have no satisfactory alternative treatments or that have progressed following treatment. In May 2020, the USFDA approved TABRECTA (Capmatinib) by Novartis Pharmaceuticals, an oral, potent, and selective MET inhibitor, the first-line therapy for patients with metastatic MET exon 14 skipping-mutated (METex14) NSCLC. Novartis licensed Capmatinib from Incyte Corporation in 2009.
Expected Hurdles in the Non-Small Cell Lung Cancer Market
A lack of available patients for trials specific to particular mutations could retard the development of therapies. Moreover, the development of therapies for specific mutations limits the eligible patient population for drugs that target the overall population.
For patients with BRAF V600E mutations, the combination of dabrafenib and trametinib is an approved treatment. However, BRAF mutations are relatively rare in NSCLC compared to other cancers, such as melanoma, which limits the number of patients who could benefit from this treatment.
Currently, TRK inhibitors have shown promising efficacy and good tolerability in patients with solid tumors that harbor NTRK fusions, regardless of the tumor’s histology. First-generation TRK inhibitors, such as larotrectinib and entrectinib, are recommended as initial treatments for patients with locally advanced or metastatic NSCLC who have confirmed NTRK fusion. However, resistance to TRK inhibitors can develop over time due to various mechanisms, either directly targeting the TRK protein or through indirect pathways. Interestingly, NTRK fusion has been identified as a potential resistance mechanism to EGFR-TKIs, suggesting that combining EGFR-TKIs with TRK inhibitors could offer a potential treatment strategy for patients experiencing resistance to EGFR-TKIs mediated by NTRK fusion.
What Lies Ahead in the Non-Small Cell Lung Cancer Market?
The development of therapies targeting specific mutations is expected to dominate the upcoming market. The total Non-Small Cell Lung Cancer market size is estimated to grow with a significant CAGR during the study period.

The premium pricing of emerging therapies is expected to create a significant advantage over existing treatments. These innovative therapies are poised to capture substantial market share with their improved clinical profiles and targeted precision for mutations specific to non-small cell lung cancer (NSCLC). The introduction of such cutting-edge treatments will not only enhance patient outcomes but also drive market growth, potentially multiplying its value as they gain traction and offer more effective solutions for those with specific genetic profiles.

Downloads
Article in PDF




