Novel Mutation-Targeting Therapies in the Horizon to Relieve the Global Healthcare Burden NSCLC Poses
Nov 29, 2024
Table of Contents
Lung cancer, to date, remains the leading cause of death worldwide, however, the epidemiological analysis depicts varying NSCLC incidence all over the world. In 2022, there were an estimated 20 million new cancer cases and 9.7 million cancer-related deaths. The number of people living for at least five years after a cancer diagnosis was estimated at 53.5 million. About 1 in 5 individuals will develop cancer in their lifetime, with approximately 1 in 9 men and 1 in 12 women succumbing to the disease.
Non-small Cell Lung Cancer Epidemiology
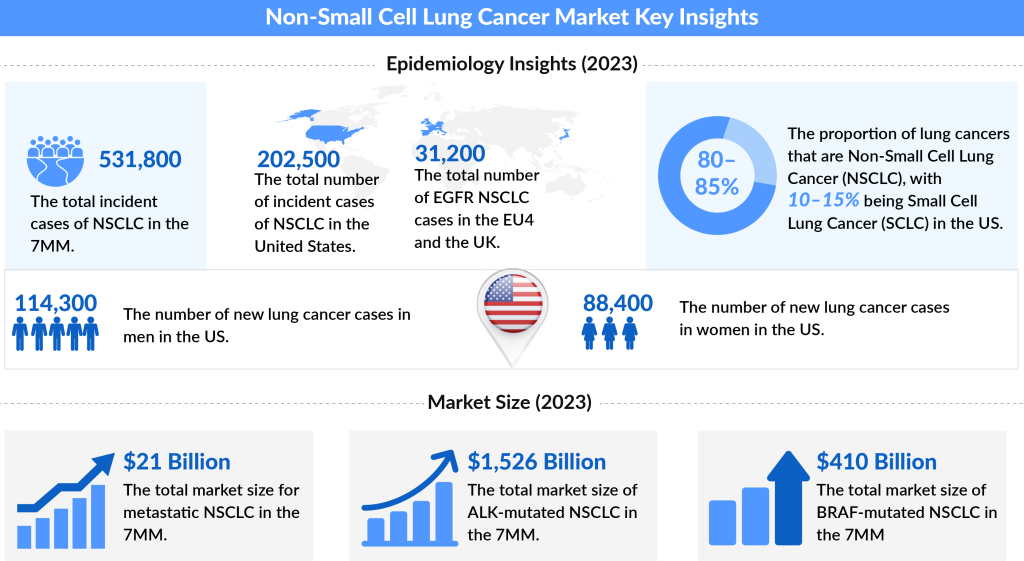
Non-small Cell Lung Cancer (NSCLC) accounts for 80-85% of all lung cancer cases, making it the most prevalent subtype. In 2023, the United States saw approximately 200K new cases of lung cancer, including 117K cases in men and 120K in women. Lung cancer remains a significant health concern, with NSCLC outpacing the less common Small Cell Lung Cancer (SCLC), which comprises 10-15% of cases.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- Abbvie-Genmab’s EPKINLY/TEPKINLY Performance in DLBCL Treatment: The First CD20XCD3 Bispecific An...
- Companion Diagnostics
- Sun Pharma forms partnership; Pfizer and DCRI collaborate; Avara Acquires AZ; ASLAN Pharmaceutica...
- Gene and Cell Therapies in CNS Disorders: Miracle Cure? Opportunities Galore!
- Sanofi invests; ICER pitches; $6M in NIH; Pfizer, Roche cancer drug pricing
NSCLC is further categorized into three main histological subtypes: adenocarcinoma, squamous cell carcinoma, and large cell (undifferentiated) carcinoma. Adenocarcinoma, originating in the glandular cells of the lung, represents around 40% of all lung cancer cases. Squamous cell carcinoma, arising from the flat cells lining the airways, accounts for 25-30%, while large cell carcinoma, an undifferentiated form, comprises approximately 10-15% of cases.
Genetic mutations and biomarkers play a crucial role in the diagnosis and treatment of NSCLC. The most frequent biomarkers include EGFR mutations (64%), ALK rearrangements (60%), PD-L1 expression (48%), ROS1 mutations (46%), and BRAF mutations (40%). Other notable biomarkers such as MET (35%), KRAS (29%), RET (22%), HER2 (21%), and PIK3CA (20%) are also significant in guiding targeted therapies. These genetic markers help tailor treatment plans, improving outcomes for patients with advanced or metastatic disease.
The epidemiology and treatment patterns of NSCLC are studied across the 7MM (United States, EU4, United Kingdom, and Japan), covering data from 2020 to 2034. This data highlights trends in incidence, histological subtypes, stages at diagnosis, and the growing impact of personalized therapies targeting genetic mutations. With a robust focus on these factors, advancements in treatment are helping redefine the standard of care for NSCLC patients worldwide.
Non-small cell lung cancer Market: Diagnosis and current therapies
Diagnosis
Non-small cell lung cancer (NSCLC) accounts for 81% of all lung cancer diagnoses, making it the most common form of lung cancer. Early diagnosis is crucial for improving prognosis. However, NSCLC is often challenging to detect in its initial stages due to symptoms that may mimic those of common respiratory illnesses or the effects of long-term smoking. Consequently, approximately 80% of NSCLC cases are diagnosed at advanced stages, making treatment more complex.
To diagnose NSCLC, physicians typically recommend imaging tests such as CT, PET, or MRI scans to identify abnormalities in and around the lungs. A sample of mucus may also be collected for microscopic examination. If these tests suggest cancer, further diagnostic procedures, including a lung biopsy or bronchoscopy, may be conducted. Bronchoscopy allows the physician to visualize lung tissues directly and collect samples for analysis.
Once NSCLC is confirmed, genetic testing of lung tissue is essential to understanding the cancer’s molecular characteristics, which can help tailor treatment options.
Current Therapies
Treatment for NSCLC depends on factors such as the cancer’s type and stage, side effects of treatment, and the patient’s overall health and preferences. Common treatment approaches include:
- Surgery
- Radiotherapy
- Chemotherapy
- Chemoradiotherapy (combination of chemotherapy and radiotherapy)
- Immunotherapy
- Targeted cancer drugs
Marketed Drugs for NSCLC
- KEYTRUDA (pembrolizumab) – Merck
KEYTRUDA, an anti-PD-1 therapy, enhances the immune system’s ability to recognize and combat tumor cells. Approved by the FDA in January 2023, it is indicated for stage IB (T2a ≥4 cm), II, or IIIA NSCLC patients after adjuvant chemotherapy. KEYTRUDA is approved for both adjuvant and metastatic settings, regardless of PD-L1 expression, making it a comprehensive option for NSCLC treatment. - LIBTAYO (cemiplimab-rwlc) – Regeneron Pharmaceuticals
LIBTAYO, a fully human monoclonal antibody targeting PD-1, was approved in November 2022 for use with platinum-based chemotherapy in first-line treatment of advanced NSCLC without EGFR, ALK, or ROS1 aberrations. It can also be prescribed as monotherapy for patients with high PD-L1 expression, expanding its utility for various patient profiles. - OPDIVO (nivolumab) – Bristol Myers Squibb
In October 2024, Opdivo was approved for resectable NSCLC without EGFR mutations or ALK rearrangements. It is used in combination with platinum-doublet chemotherapy as a neoadjuvant treatment and as monotherapy after surgery. - TAGRISSO (osimertinib) – AstraZeneca
Approved in September 2024, TAGRISSO treats unresectable Stage III EGFR-mutated NSCLC in patients without disease progression after chemoradiation therapy. It significantly prolongs progression-free survival for patients with specific EGFR mutations. - RYBREVANT (amivantamab-vmjw) – Johnson & Johnson
RYBREVANT was approved in 2024 for use with standard chemotherapy in patients with EGFR exon 19 deletions or L858R substitution mutations. Another approval includes its use with LAZCLUZE™ for first-line treatment in patients with advanced NSCLC harboring these genetic abnormalities. - IMFINZI (durvalumab) – AstraZeneca
Approved in August 2024, Imfinzi is used with chemotherapy for early-stage NSCLC (IIA-IIIB) without EGFR mutations or ALK rearrangements. This regimen includes treatment before surgery and adjuvant monotherapy afterward, reducing recurrence, progression, or death by 32%.
These advancements underscore the growing arsenal of targeted therapies and immunotherapies in the NSCLC market, improving survival outcomes and addressing diverse patient needs.
In the NSCLC Pipeline
At present, there are several emerging therapies in the Non-small cell lung cancer market are Zipalertinib, YK-029A, TAS3351, NVL-330, JS-113, JANX-008, IN-119873, and others which target different types of mutations and form the major NSCLC treatment option in the Non-small cell lung cancer market.
Even though the Non-small cell lung cancer market constitutes a robust pipeline, the cost-effectiveness of targeted therapies comes out as a great dejecting factor which is a major barrier in the growth of non-small cell lung cancer market. Key players such as Merck & Co., Inc., Novartis AG, Pfizer Inc., Takeda Pharmaceutical, Bayer AG, F. Hoffmann-La Roche Ltd., AstraZeneca, Bristol-Myers Squibb Company, Eli Lilly and Company, GlaxoSmithKline plc., Sanofi, Agennix AG, and others are involved in developing drugs for Non-small cell lung cancer (NSCLC) that are responsible for the change in dynamics of the Non-small cell lung cancer (NSCLC) market by 2032. In addition to the key companies, hikes in healthcare spending, and early diagnosis due to a better understanding of the disease are other factors driving the NSCLC market forward.

Downloads
Article in PDF
Recent Articles
- The Business Cocktail
- Pfizer’s COVID-19 Vaccine; Calliditas’ NefIgArd trial; ViiV’s HIV Prevention Tr...
- Idera’s drug; CRISPR increase risk; Juvenescence grabbed USD 50M; AstraZeneca, Lilly termin...
- Merck Wins FDA Approval for KEYTRUDA QLEX for Subcutaneous Use in Adults With Solid Tumors; Incyt...
- Nordisk ties up with Dicerna; Pfizer’s drug gets FDA nod