Evaluating Key Advancements and Emerging Therapies in EGFR-Non Small Cell Lung Cancer Treatment Market
Nov 29, 2024
Table of Contents
Non-small cell lung cancer (NSCLC) is the most common cancer, accounting for approximately 85% of the total lung cancers in the United States. Almost 500K cases in the 7MM were diagnosed in 2022 alone. Moreover, significant deaths were recorded at the end of the same year. Analysis indicates that the most common genetic alteration in Non-Small Cell Lung Cancer (NSCLC) is the EGFR mutation, which occurs in approximately 20–25% of patients. These mutations primarily involve exon 19, where a deletion occurs, and exon 21, specifically the L858R mutation. Together, these mutations account for about 80–85% of all EGFR mutations in NSCLC.
Non-small cell lung cancer is any type of epithelial lung cancer other than SCLC. It is further subcategorized into adenocarcinomas, squamous cell carcinomas, and large cell carcinomas. There are other types occurring less frequently, including adenosquamous carcinomas and sarcomatoid carcinomas.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- Notizia
- Takeda and AC Immune’s Alzheimer’s Deal; Eli Lilly’s Donanemab FDA Review; Bristol Myers Sq...
- BGB-16673 Breakthrough: A Tolerable Triumph in Rapid Clinical Responses for Relapsed/Refractory B...
- FDA Approves PTC’s AADC Gene Therapy; DUPIXENT sBLA Acceptance for Urticaria; CHMP Recommen...
- FDA Approval to DePuy TELIGEN System; FDA Breakthrough Device Designation for the EndoStim System...
There are several genetic mutations that play a major role in the progression of the indication. EGFR, KRAS, and ALK Mutations are mutually exclusive in patients with NSCLC. Moreover, the presence of one mutation instead of another can influence the response to targeted therapy. Therefore, testing for these mutations and tailoring therapies accordingly is widely accepted as standard practice.
EGFR is a member of the ErbB family of receptor tyrosine kinases (RTK). Activation of the receptor initiates a cascade of downstream signaling molecules, leading to cell growth and survival. This process is tightly regulated in non-transformed cells. However, constitutive activation of these pathways leads to dysregulated cell growth and proliferation, angiogenesis, and metastasis, while apoptosis is repressed. Genomic damage can introduce mutations that mimic kinase activation or result in higher expression levels of EGFR.
Currently, several treatment options are available to treat EGFR Non-Small Cell Lung Cancer in the seven major markets. Typically, chemotherapy, targeted treatments, and immunotherapy alone or in combination are used to treat lung cancer. The trending therapies in the Non-small cell lung cancer market include Telisotuzumab Vedotin, Datopotamab deruxtecan, and various other therapies are being used widely to treat the ailment. The disease lacks permanent cures, and therefore, several key players like Dizal Pharmaceuticals, Novartis Pharmaceuticals, AstraZeneca, Betta Pharmaceuticals Co., Ltd., Hangzhou ACEA Pharmaceutical Research, G1 Therapeutics, Inc., Millennium Pharmaceuticals, Inc., Takeda, Janssen Research & Development, LLC, Sinocelltech Ltd., Allist Pharmaceuticals, Inc., and many others are working to bring novel therapies that overcome the roadblocks of existing therapies.
Standard therapy, namely epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKI), has been developed for treating EGFR-sensitizing mutant advanced non-small-cell lung cancer (NSCLC). However, non small cell lung cancer treatment patients frequently develop resistance to most drugs, including EGFR-TKI. Let’s understand more about it.

Resistance to Old-Generation EGFR TKIs
There are several primary and secondary resistance mechanisms for EGFR-TKIs in EGFR-non small cell lung cancer treatment market. The primary resistance mechanisms include point mutations in exon 18, deletions or insertions in exon 19, insertions, duplications, point mutations in exon 20, and point mutation in exon 21 of the EGFR gene. The secondary resistance to EGFR-TKIs is majorly seen due to the emergence of T790M mutation, activation of alternative signaling pathways, bypassing downstream signaling pathways, and histological transformation.
The basic strategies for overcoming the intrinsic and acquired resistance mechanisms are quite complex. With the advancements in precision medicine for advanced NSCLC treatment, various local and systemic treatment options have been expanded. These options, in turn, need new clinical algorithms that note the cancerous cells’ resistance mechanism. In general, combination therapies have been used to cope with the frequently emerging resistance.
Apart from that, various customized treatment plans and regimens have been created for the patient based on molecular diagnosis and monitoring. The development and finding from ongoing clinical trials suggest that combination therapy using third-generation TKIs and antibodies in EGFR mutant NSCLC is promising for better survival outcomes.
For a better understanding of the ailment, comprehensive biomarker testing techniques have been developed to determine EGFR lung cancer mutations and other lung cancer mutations.
While getting diagnosed with NSCLC, patients should ensure that their doctors have ordered comprehensive biomarker testing on their lung cancer tumor. Also, they should get results beforehand and then proceed with other EGFR-non-small cell lung cancer treatment, including chemotherapy and immunotherapy. A comprehensive biomarker testing includes a lung cancer biopsy in which cancer cells are extracted, and their genetic makeup and biomarkers are examined.
Market Assessment and Reimbursement
In January 2020, it was announced that Tagrisso is not recommended, within its marketing authorization, for untreated locally advanced or metastatic EGFR mutation-positive NSCLC in adults in the UK. This recommendation is not intended to affect EGFR-non small cell lung cancer treatment with osimertinib, which was started in the NHS before this guidance was published. People having treatment outside this recommendation may continue without change to the funding arrangements in place for them before this guidance was published until they and their NHS clinician consider it appropriate to stop.
In addition to this, in September 2016, TAGRISSO received an Unfavorable decision from IQWiG (Germany) for Locally advanced or metastatic EGFR T790M mutation-positive NSCLC. Furthermore, contrary to the above-mentioned HTA bodies, Haute Autorité de santé (HAS) France announced a favorable decision for TAGRISSO in September 2016. In Sept 2024, the European Medicines Agency’s CHMP has recommended AstraZeneca’s TAGRISSO (osimertinib) for approval in the EU to treat locally advanced, unresectable non-small cell lung cancer (NSCLC). Before that, in July 2024, AstraZeneca received Health Canada’s Notice of Compliance for TAGRISSO, in combination with pemetrexed and platinum-based chemotherapy, to treat EGFR-mutated lung cancer.
Emerging Therapies of EGFR-NSCLC
Besides this, Abbvie is investigating their drug candidate, Telisotuzumab vedotin (ABBV-399), an antibody-drug conjugate targeting c-Met, a tyrosine receptor kinase that is overexpressed in NSCLC tumors. Currently, the drug has no regulatory approvals, and studies to determine its safety and efficacy are underway. In January 2022, Abbvie announced that US FDA had granted Breakthrough Therapy Designation to investigational telisotuzumab vedotin (Teliso-V) for treating patients with advanced/metastatic EGFR wild type NSCLC. As per the presentation, this is the first Breakthrough Therapy Designation for an AbbVie for solid tumor investigational therapy. Topline results from the Phase II LUMINOSITY trial of telisotuzumab vedotin (Teliso-V) in patients with c-Met overexpression and EGFR wild type advanced/metastatic nonsquamous NSCLC showed an overall response rate of 35% in c-Met High patients and 23% in c-Met Intermediate patients, as per independent central review (ICR).
Telisotuzumab vedotin (Teliso-V) is currently in preregistration for non-small cell lung cancer. In September 2024, AbbVie announced the submission of a Biologics License Application (BLA) to the FDA, seeking accelerated approval for Teliso-V in adult patients with previously treated, locally advanced or metastatic, nonsquamous non-small cell lung cancer (NSCLC) with EGFR wild type and c-Met protein overexpression.
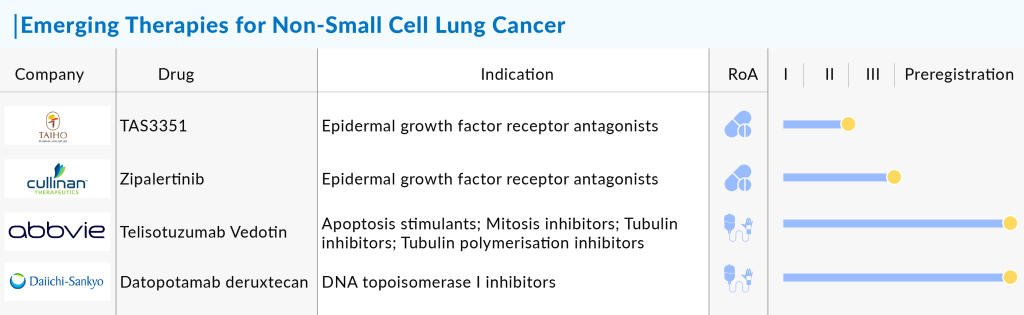
Companies like Dizal Pharmaceuticals (DZD9008; Sunvozertinib), Cullinan Oncology (CLN-081), Epimab BioTherapeutics (EMB-01), and several others are also evaluating their products in EGFR-NSCLC. Dizal’s DZD9008 received breakthrough designation from the US FDA to treat patients with locally advanced or metastatic NSCLC with EGFR exon20 insertion mutations. In April 2024, Dizal announced that the FDA granted Breakthrough Therapy Designation (BTD) to sunvozertinib for first-line treatment of patients with locally advanced or metastatic NSCLC with EGFR exon 20 insertion mutations.
Furthermore, In August 2023, data from a Phase 1/2a trial (NCT04036682) published in the Journal of Clinical Oncology showed that Zipalertinib (CLN-081, TAS6417) demonstrated promising efficacy and an acceptable safety profile in heavily pretreated patients with recurrent or metastatic NSCLC harboring EGFR exon 20 insertions. Apart from this, some companies’ drugs are in the early or preclinical phases, such as RJS Biologics, Scorpion Therapeutics, KANAPH Therapeutics, and others.
Developments and Advancements in EGFR-NSCLC Domain
In July 2015, the US FDA approved IRESSA (gefitinib) for the first-line treatment of patients with NSCLC whose tumor harbor specific types of EGFR gene mutations, as detected by an FDA-approved test. However, before a nod from the US FDA, in July 2009, European Medicines Agency (EMA) granted marketing authorization for gefitinib to treat locally advanced or metastatic NSCLC with sensitizing mutations of the EGFR gene across all lines of therapy.
In September 2018, the US FDA approved Pfizer’s VIZIMPRO (dacomitinib), a kinase inhibitor for the first-line EGFR-non small cell lung cancer treatment of patients with metastatic NSCLC with EGFR exon 19 deletion or exon 21 L858R substitution mutations as detected by an FDA-approved test. Earlier this, the FDA granted Priority Review for VIZIMPRO for the first-line EGFR-non small cell lung cancer treatment of patients with locally advanced or metastatic NSCLC with EGFR-activating mutations.
Recently, in May 2021, the US FDA granted the accelerated approval of RYBREVANT (amivantamab-vmjw, Janssen) for the EGFR-non small cell lung cancer treatment of adult patients with locally advanced or metastatic NSCLC with EGFR exon 20 insertion mutations, as detected by an FDA-approved test, whose disease has progressed on or after platinum-based chemotherapy. RYBREVANT is the first fully human, bispecific antibody approved for treating patients with NSCLC that targets EGFR exon 20 insertion mutations. This approval follows the FDA’s decision to grant Breakthrough Therapy Designation in March 2020 and to initiate a Priority Review of the Biologics License Application in December 2020. Moreover, the US FDA simultaneously approved Guardant Health’s Guardant360 CDx liquid biopsy blood test as a companion diagnostic for use with RYBREVANT.
In September 2021, US FDA approved EXKIVITY (Mobocertinib, Takeda) to treat adult patients with locally advanced or metastatic NSCLC. It is also the first and only approved oral therapy specifically designed to target EGFR Exon20 insertion mutations. This indication is approved under Accelerated Approval based on the overall response rate (ORR) and Duration of response. EXKIVITY is a kinase inhibitor indicated for treating adult patients with locally advanced or metastatic NSCLC with epidermal growth factor receptor (EGFR) exon 20 insertion mutations, as detected by an FDA-approved test, whose disease has progressed on or after platinum-based chemotherapy.
In January 2022, the AK112 trial was initiated to treat NSCLC. The trial will be performed as a randomized, double-blind, multicenter study to compare AK112 plus pemetrexed and carboplatin to placebo plus pemetrexed and carboplatin in patients with locally advanced or metastatic non-squamous non-small cell lung cancer (NSCLC).
AK112 plus platinum-doublet showed promising antitumor activity and safety not only in first-line treatment of advanced NSCLC patients without driver mutations but also in patients with EGFR mutations who failed previous EGFR-TKI therapy, as well as in advanced NSCLC patients who failed prior systemic platinum-based chemotherapy and PD-1/PD-L1 inhibitor treatments. These findings suggest that AK112 could offer a valuable new treatment option for these patient populations. In addition, the combination of carboplatin in EGFR-non-small cell lung cancer treatment periods demonstrated significant clinical benefits, reinforcing its potential in diverse treatment settings. In conclusion, AK112 combined with chemotherapy demonstrated promising antitumor activity and was well-tolerated in patients with advanced NSCLC, indicating its potential as a valuable new treatment option for this group.
NSCLC is still one of the major global concerns due to its high incident patient population and death rates. There is currently a list of approved NSCLC treatment drugs; however, a patient population with unknown mutations may not benefit significantly from the available EGFR-non small cell lung cancer treatment choices. There is a scope for improvement and major medical unmet needs associated with NSCLC.
Entry of Tagrisso in the First-line setting and Evolving EGFR-Non Small Cell Lung Cancer Treatment Landscapes for EGFR Exon 20 Mutations in NSCLC
The present market of EGFR-positive NSCLC is mainly dominated by TAGRISSO (osimertinib); other EGFR-non small cell lung cancer treatment regimens like platinum-based chemotherapy, first and second generations TKIs and VIZIMPRO (dacomitinib) are also contributing to the market size. The upcoming therapeutic market is expected to be propelled by agents that are targeting EGFR exon 20 insertion mutations like TAK-788 and others. However, TAGRISSO will remain the standard of care and is also expected to dominate the market. The EGFR exon 20 mutation is an uncommon mutation of EGFR mutation class, but it is very difficult to treat and is a major cause of resistance.
Approximately 60% of acquired resistance to the first generation of EGFR-TKIs results from EGFR exon 20 T790 M mutations. In May, the FDA granted accelerated approval to RYBREVANT for patients with locally advanced or metastatic NSCLC harboring EGFR exon 20. RYBREVANT was granted expedited approval by the FDA in May for patients with locally advanced or metastatic NSCLC having EGFR exon 20. RYBREVANT is one of a growing number of medicines in clinical trials that have an action against EGFR exon 20 insertion–mutant disease.
The previously untreated EGFR mutation-positive metastatic NSCLC was demonstrated in a randomized, multicenter, double-blind, active-controlled trial (FLAURA [NCT02296125]). The use of osimertinib in the frontline setting had increased quite rapidly in the few months after the FLAURA data were released and was expected to continue for a while.
In conclusion, the market will proliferate with the expected launch of emerging and present therapies.
EGFR-NSCLC Therapeutics Market Assessment – Future Analysis and Perspective
Treatment of non-small cell lung cancer (NSCLC) harboring epidermal growth factor receptor (EGFR) activating mutation with EGFR-TKIs has achieved great success, yet faces the development of acquired resistance as the major obstacle to long-term disease remission in the clinic. MET (or c-MET) gene amplification has long been known as an important resistance mechanism to first- or second-generation EGFR-TKIs in addition to the appearance of T790 M mutation. Recent preclinical and clinical studies have suggested that MET amplification and protein hyperactivation is likely to be key mechanism underlying acquired resistance to third-generation EGFR-TKIs such as osimertinib, particularly when used as a first-line therapy. EGFR-mutant NSCLCs that have relapsed from first-generation EGFR-non small cell lung cancer treatment and have MET amplification and protein hyperactivation should be insensitive to osimertinib monotherapy. Therefore, combinatorial therapy with osimertinib and MET or even a MEK inhibitor should be considered for these patients with resistant NSCLC carrying MET amplification and protein hyperactivation.
The discovery of druggable protein kinases that mediate several aberrant tumorigenic processes and signal transduction pathways has led to a revolutionary change in EGFR-non small cell lung cancer treatment. Epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase (ALK) are the targets of several tyrosine kinase inhibitors (TKIs), some of them approved for the treatment and others currently in clinical development. First-generation agents offer, in target populations, a substantial improvement of outcomes compared with standard chemotherapy in the advanced EGFR-non small cell lung cancer treatment. Unfortunately, drug resistance develops after initial benefit through a variety of mechanisms.
Novel generation EGFR and ALK inhibitors are currently in advanced clinical development and are producing encouraging results in patients with acquired resistance to previous-generation agents. The search for new drugs or strategies to overcome TKI resistance in patients with EGFR mutations or ALK rearrangements is to be considered a priority for improving outcomes in advanced EGFR-non small cell lung cancer treatment. Potential emerging therapies with better clinical profiles and specificity towards mutations occurring in NSCLC can dominate the EGFR-NSCLC market and call offer sufficient space for new key players.

Downloads
Article in PDF
Recent Articles
- Dizal’s ZEGFROVY Approval Heating Up EGFR NSCLC Drug Rivalry with J&J
- Generative AI in Drug Discovery: Applications and Market Impact
- Notizia
- ENHERTU’s Rapid Rise: How the HER2-Targeting ADC Is Redefining Cancer Treatment with Back-to-Back...
- How HR+/ HER2-Breast Cancer Emerging Drugs Will Transform The Market?



