Molecular Glues Market Summary
- The Molecular Glues market size is expected to grow rapidly with a significant CAGR during the study period (2020–2034).
- Molecular glue compounds are a type of unique small molecule that can change the protein–protein interactions (PPIs) and interactomes by degrading, stabilizing, or activating the target protein after their binging.
- Molecular glues offer a novel approach to target traditionally undruggable proteins like β-catenin, providing new avenues for therapeutic intervention.
- PROTAC and molecular glue are the most advanced targeted protein degradation (TPD) technology. Both are based on the ubiquitin-proteasome system and useful for the degradation of intracellular proteins.
- Monte Rosa is also immersed in the field of molecular glue degraders. Apart from its collaboration with Roche, it is building an oncology pipeline of its own. Most advanced in its lineup is its clinical candidate MRT-2359, which is an oral molecular glue.
- The market for molecular glues is expanding. Approved molecular glues found in the market include POMALYST (Bristol Myers Squibb), REVLIMID (Bristol Myers Squibb), MEKINIST (Novartis), and many more.
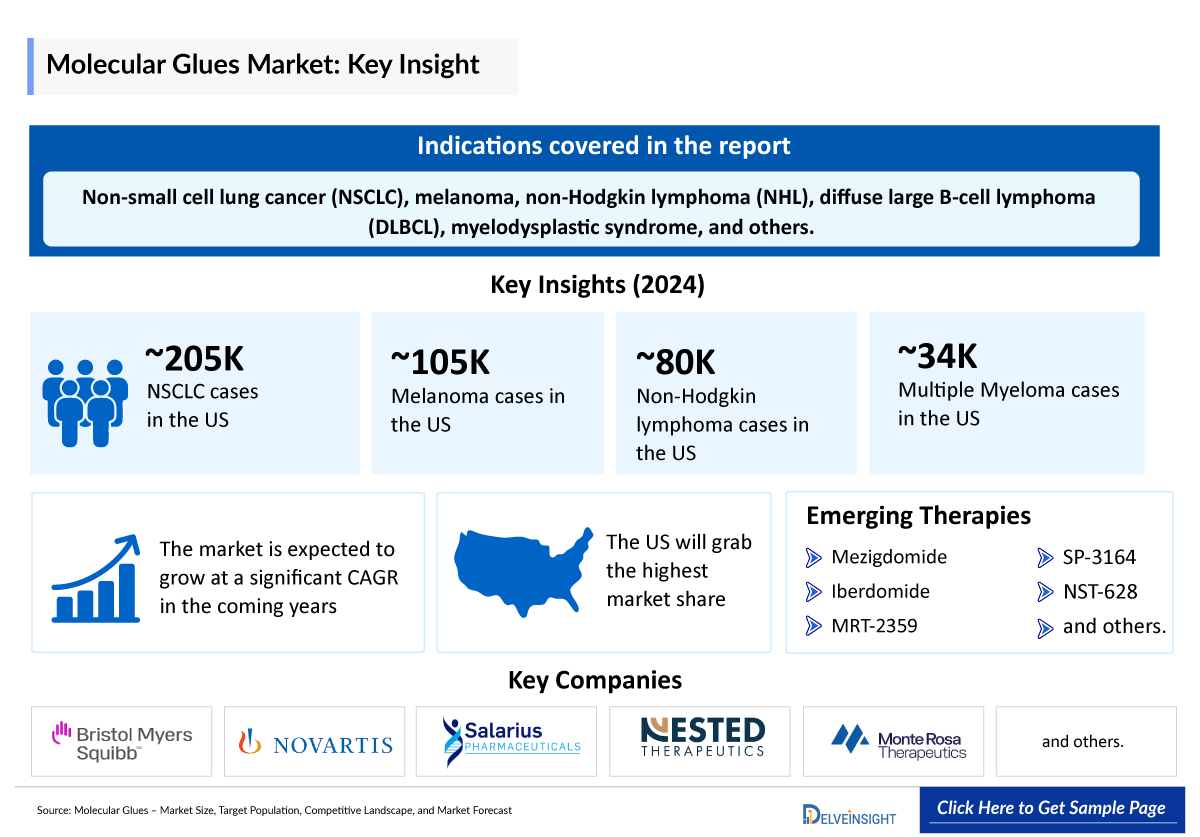
- The emerging drug pipeline for molecular glues targeted therapies is diverse and includes several promising candidates, such as Mezigdomide (Bristol Myers Squibb), Iberdomide (Bristol Myers Squibb), SP-3164 (Salarius Pharmaceuticals), NST-628 (Nested Therapeutics), MRT-2359 (Monte Rosa Therapeutics), and others.
- The molecular glue therapy landscape is evolving, driven by technological advancements aimed at reducing toxicity, targeting specific receptors, and combining with other immunotherapies (e.g., checkpoint inhibitors). As the pipeline continues to mature, molecular glue based therapies are expected to transform the treatment of cancer, autoimmune diseases, and inflammatory disorders.
Factors Driving Molecular Glues Market Growth
Rising Focus on Targeted Protein Degradation (TPD)
- Molecular glues represent an advanced approach within the Targeted Protein Degradation (TPD) field, enabling the selective degradation of disease-causing proteins that were previously considered “undruggable.”
- The growing scientific and commercial interest in TPD is fueling substantial R&D investment, accelerating the adoption of molecular glues as a novel therapeutic class.
Expanding Research Pipeline and Collaborations
- Increasing collaborations between biopharmaceutical companies, academic institutes, and biotech start-ups are fostering innovation in molecular glue discovery and optimization.
- Several partnerships (e.g., between Arvinas, C4 Therapeutics, Monte Rosa Therapeutics, and major pharma companies) are driving early-stage development and clinical validation.
Ability to Target Undruggable Proteins
- Traditional small-molecule drugs can only target around 10–15% of the human proteome, while molecular glues offer a new therapeutic strategy to modulate previously inaccessible targets.
- This expanded scope significantly increases market potential, especially for oncology, neurodegenerative, and autoimmune indications.
Increasing Preclinical and Clinical Successes
- Positive preclinical results and advancing clinical trials are building confidence in the molecular glue mechanism, drawing investor attention and stimulating funding.
- Examples include compounds modulating E3 ligases for the degradation of transcription factors and oncogenic proteins.
Rising Investment and Venture Capital Funding
- Venture capital and strategic investment in molecular glue-focused biotech firms are rapidly growing, driven by the promise of first-in-class and disease-modifying therapies.
- This influx of capital accelerates discovery platforms, AI-driven screening, and commercialization readiness.
Advances in Structural Biology and AI-Based Drug Discovery
- Progress in AI, computational chemistry, and proteomics has significantly improved the identification and design of molecular glues.
- Structural insights into E3 ligases and protein–protein interactions enable more efficient screening and rational design, enhancing R&D productivity.
Growing Oncology and Rare Disease Applications
- Molecular glues show strong potential in oncology, hematologic malignancies, and rare genetic disorders, where targeted degradation of specific proteins offers superior therapeutic outcomes compared to inhibition alone.
- Expanding application areas are diversifying market opportunities.
Strategic Partnerships with Big Pharma
- Large pharmaceutical companies are increasingly entering collaborations or licensing deals to access molecular glue platforms, validating the commercial and therapeutic potential of this modality.
- These alliances provide funding, infrastructure, and regulatory expertise, speeding up clinical development.
Supportive Regulatory Environment and Scientific Recognition
- Regulatory bodies and scientific communities are showing growing recognition of Targeted Protein Degradation therapies, paving the way for smoother clinical advancement and regulatory pathways.
- Publications and conferences on TPD are increasing year over year, indicating a strong global research ecosystem.
Potential for Combination Therapies
Molecular glues can be combined with existing targeted therapies, immunotherapies, or kinase inhibitors, opening avenues for synergistic treatment approaches and expanding clinical use cases.
DelveInsight’s “Molecule Glues Market Size Target Population, Competitive Landscape, and Market Forecast – 2034” report delivers an in-depth understanding of the molecule glues, historical and Competitive Landscape as well as the molecule glues market trends in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
The molecule glues market report provides current treatment practices, emerging drugs, market share of individual therapies, and current and forecasted 7MM molecule glues market size from 2020 to 2034. The report also covers current molecule glues treatment practices/algorithms and unmet medical needs to curate the best opportunities and assess the market’s potential.
Scope of the Molecule Glues Market Report | |
|
Study Period |
2020-2034 |
|
Forecast Period |
2025-2034 |
|
Geographies Covered |
US, EU4 (Germany, France, Italy, and Spain) the UK, and Japan |
|
Molecule Glues Epidemiology |
Segmented by:
|
|
Molecule Glues Key Companies |
|
|
Molecule Glues Key Therapies |
|
|
Molecule Glues Market |
Segmented by:
|
|
Analysis |
|
Molecule Glues Understanding
Molecule Glues Overview
Molecular glues are a special class of small molecules that can influence how proteins interact with each other. By binding to target proteins, they can trigger their degradation, stabilization, or activation. Along with PROTACs (PROteolysis Targeting Chimera), molecular glues represent the most advanced technologies in targeted protein degradation (TPD), both working through the cell’s natural ubiquitin-proteasome system to break down unwanted proteins inside cells.
One of the key advantages of molecular glues is their ability to target proteins that were previously considered "undruggable," such as β-catenin, opening up new possibilities for treating diseases. They also tend to have favorable pharmacokinetic profiles, making them promising drug candidates. However, only a few well-studied molecular glues are currently known, and researchers still lack a clear understanding of how they work or how to design them effectively. As a result, more in-depth research is needed to fully unlock their potential.
Further details related to country-based variations are provided in the report
Molecule Glues Epidemiology
The molecule glues epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented as total cases of selected indications for molecule glues, indication-wise eligible cases for molecule glues in selected indication, indication-wise treated cases in selected indication for molecule glues in the 7MM covering the United States, EU4 (Germany, France, Italy, and Spain), and the United Kingdom, and Japan from 2020 to 2034.
Estimated Total Cases of Selected Indications in the US in 2024 | ||
|
S.No. |
Indications |
Total Cases |
|
1 |
∼205,000 | |
|
2 |
∼105,000 | |
|
3 |
∼80,300 | |
|
4 |
∼33,700 | |
|
5 |
∼31,500 | |
|
6 |
∼15,500 | |
|
7 |
∼5,300 | |
NOTE: The list of indications is not exhaustive, and will be provided in the final report. Also, numbers are indicative and are subject to change as per report updation
Molecule Glues Drugs Analysis
The drug chapter segment of the molecule glues reports encloses a detailed analysis of molecule glues-marketed drugs and late-stage (Phase III and Phase II) pipeline drugs. It also helps understand the molecule glues clinical trial details, expressive pharmacological action, agreements and collaborations, approval and patent details, advantages and disadvantages of each included drug, and the latest news and press releases.
Molecule Glues Marketed Drugs
POMALYST (pomalidomide): Bristol Myers Squibb
POMALYST (pomalidomide) is a cancer medicine used to treat multiple myeloma. It is used in combination with bortezomib and dexamethasone in adults who have received at least one treatment including lenalidomide. It is also used in combination with dexamethasone in adults who have received at least two prior therapies, including both lenalidomide and bortezomib, and whose disease has worsened. It was approved for the treatment of multiple myeloma by the US FDA in 2013. The first generic medicine for POMALYST was approved by the FDA in 2024.
REVLIMID (lenalidomide): Bristol Myers Squibb
REVLIMID (lenalidomide) is an oral immunomodulatory medication approved by the FDA in 2006 for the treatment of multiple myeloma. Over the years, it has gained multiple approvals, including as part of combination regimens and as maintenance therapy following autologous stem cell transplant (ASCT).
REVLIMID became a blockbuster drug due to its strong clinical efficacy, broad use across different stages of multiple myeloma, and strategic lifecycle management through expanded indications. At its peak, the drug generated over USD 12 billion in annual sales globally, becoming one of the best-selling oncology drugs of all time. However, REVLIMID started facing generic competition in the US from March 2022. As a result, its sales have been steadily declining as more patients switch to lower-cost generic versions.
Molecule Glues Emerging Drugs
Mezigdomide: Bristol Myers Squibb
Mezigdomide is a highly potent modulator of the E3 ubiquitin ligase complex containing cereblon (CRL4-CRBN E3 ubiquitin ligase), with potential immunomodulating and antineoplastic activities. The company is evaluating the drug in different clinical trials in different lines of therapies for treating NDMM and RRMM.
- The company is expecting the first approval of mezigdomide by 2026–2027.
- The company anticipated the data readout for Phase III – SUCCESSOR-1 and Phase III – SUCCESSOR-2 trials by 2026.
- In June 2025, Bristol Myers Squibb (BMS) shared new clinical data at EHA on its CELMoD agents (mezigdomide, iberdomide, golcadomide) and its first-in-class oral BCL6 ligand-directed degrader (BMS‑986458) for hematologic malignancies.
NST-628: Nested Therapeutics
NST-628, a mechanistically novel, fully brain penetrant non-degrading pan-RAF/MEK molecular glue, works by targeting the RAF and MEK nodes in the RAS-MAPK pathway. In a preclinical trial, NST-628, given as either a single agent or in combination regimens, demonstrated antitumor activity and tolerability.
- In March 2024, the FDA granted clearance to the IND application for NST-628 for the treatment of patients with advanced solid tumors with genetic alterations in the RAS-MAPK pathway. The estimated study completion date is November 2029.
MRT-2359: Monte Rosa Therapeutics
MRT-2359 is a potent, selective, and orally bioavailable investigational molecular glue degrader (MGD) that induces the interaction between the E3 ubiquitin ligase component cereblon and the translation termination factor GSPT1, leading to the targeted degradation of GSPT1 protein. The MYC transcription factors (c-MYC, L-MYC, and N-MYC) are well-established drivers of human cancers that maintain high levels of protein translation, which is critical for uncontrolled cell proliferation and tumor growth.
- In March 2025, Monte Rosa Therapeutics presented new data on MRT-2359, highlighting early clinical activity in castration-resistant prostate cancer (CRPC) and announcing plans to prioritize its development for this indication.
In December 2024, Monte Rosa Therapeutics announced that results from dose escalation arms of Phase I/II study of MRT-2359 demonstrated a favorable safety profile and targeted levels of GSPT1 degradation using a 21 days on, 7 days off drug dosing schedule in heavily pretreated solid tumor patients.
Comparison of Key Emerging Molecule Glues | ||||
|
Product |
Company |
RoA |
Phase |
Indication |
|
Mezigdomide |
Bristol Myers Squibb |
Oral |
III |
Multiple Myeloma |
|
Iberdomide |
Bristol Myers Squibb |
Oral |
III |
Multiple Myeloma |
|
MRT-2359 |
Monte Rosa Therapeutics |
Oral |
I/II |
Diffuse large B cell lymphoma, Non-small cell lung cancer |
|
SP-3164 |
Salarius Pharmaceuticals |
Oral |
I |
Non-Hodgkin's lymphoma |
|
NST-628 |
Nested Therapeutics |
Oral |
I |
Solid tumors |
Note: Detailed emerging therapies assessment will be provided in the final report.
Molecule Glues Market Outlook
While many potential molecular glue compounds may come from drug discovery methods, few have progressed to the clinic for examination of their disease treating efficacy. Regulatory agency-approved molecular glue treatments that have progressed to the market are even fewer. Companies and research organizations with a focus on molecular glue drug discovery are creating pipelines of therapeutics that are progressing to the clinic., and the increasing number of molecule glues that are under clinical trials.
Molecule Glues offer a promising therapeutic avenue across a spectrum of diseases, ranging from various cancers like non-small cell lung cancer, castration-resistant prostate cancer, and other diseases.
Bristol Myers Squibb are developing molecular glue compounds that have progressed to the market. Molecular glue therapeutics POMALYST (pomalidomide), which is indicated in the treatment of multiple myeloma and REVLIMID (lenalidomide), for the treatment of multiple myeloma, myelodysplastic syndrome, and mantle cell lymphoma, were approved by the US FDA in 2013 and 2017, respectively. Novartis is another company with an approved molecular glue. MEKINIST (trametinib), currently on the market for the treatment of melanoma, was approved by the US FDA in 2017.
A few key players, including Bristol Myers Squibb, Nested Therapeutics, Monte Rosa Therapeutics, and others are involved in developing drugs for Molecule Glues for various indications such as non-small cell lung cancer (NSCLC), melanoma, non-hodgkin lymphoma (NHL), diffuse large B-cell lymphoma (DLBCL), myelodysplastic syndrome, and others. Overall, this is an exciting new class of agents with great potential for development. The maturation of current studies over the next few years will lead to a better understanding of molecule glues and define their role in the therapy of cancer.
Molecule Glues Competitive Landscape
This section focuses on the uptake rate of potential emerging molecule glues expected to be launched in the market during 2025–2034.
Molecule Glues Companies
- Bristol Myers Squibb
- Revolution Medicines, Inc.
- Eisai Co Ltd
- Gluetacs Therapeutics (Shanghai) Co., Ltd.
- Captor Therapeutics
- GluBio Therapeutics
- Nurix Therapeutics, Inc.
- SEED Therapeutics, Inc.
- MindRank Ltd
- Monte Rosa Therapeutics
- Beijing InnoCare Pharma Tech Co., Ltd.
- C4 Therapeutics, Inc.
- Erasca, Inc.
- Plexium, Inc.
- Novartis AG
- Salarius Pharmaceuticals, Inc
- Orionis Biosciences
- Rapafusyn Pharmaceuticals
Molecule Glues Clinical Trial Analysis
The Molecule Glues market report provides insights into different therapeutic candidates in Phase III, Phase II, and Phase I. It also analyzes key Molecule Glues companies involved in developing targeted therapeutics.
The presence of numerous drugs under different stages is expected to generate immense opportunity for molecule glues market growth over the forecast period.
Molecule Glues Pipeline Development Activities
The Molecule Glues market report covers information on collaborations, acquisitions and mergers, licensing, and patent details for molecule glues therapies.
Molecule Glues Market Recent Developments and Breakthroughs
- In June 2025, Bristol Myers Squibb (BMS) shared new clinical data at EHA on its CELMoD agents (mezigdomide, iberdomide, golcadomide) and its first-in-class oral BCL6 ligand-directed degrader (BMS‑986458) for hematologic malignancies.
- In March 2025, Monte Rosa Therapeutics presented new data on MRT-2359, highlighting early clinical activity in castration-resistant prostate cancer (CRPC) and announcing plans to prioritize its development for this indication.
- In March 2024, the FDA granted clearance to the IND application for NST-628 for the treatment of patients with advanced solid tumors with genetic alterations in the RAS-MAPK pathway. The estimated study completion date is November 2029.
Latest KOL Views on Molecule Glues
To keep up with current and future market trends, we take Industry Experts’ opinions working in the domain through primary research to fill the data gaps and validate our secondary research. Industry experts were contacted for insights on molecule glues evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, drug uptake, along challenges related to accessibility.
DelveInsight’s analysts connected with 20+ KOLs to gather insights; however, interviews were conducted with 10+ KOLs in the 7MM Universities such as University of California and others.
Their opinion helps understand and validate current and emerging therapy treatment patterns or molecule glues market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
What KOLs are saying on Molecule Glues Patient Trends? |
|
“The beauty of molecular glues lies in their ability to rewire protein networks, especially in cancers driven by transcription factors or scaffolding proteins. Our group is developing chemical biology tools to discover glue-like compounds through phenotypic screens, targeting proteins once thought inaccessible.” Phd, University of California, US |
Molecule Glues Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT analysis and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of gaps in disease diagnosis, patient awareness, physician acceptability, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided.
Conjoint Analysis analyzes multiple approved and emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy.
In efficacy, the trial’s primary and secondary outcome measures are evaluated; for instance, in event-free survival, one of the most important primary outcome measures is event-free survival and overall survival.
Further, the therapies’ safety is evaluated wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Molecule Glues Market Access and Reimbursement
Reimbursement may be referred to as the negotiation of a price between a manufacturer and payer that allows the manufacturer access to the market. It is provided to reduce the high costs and make the essential drugs affordable. Health technology assessment (HTA) plays an important role in reimbursement decision-making and recommending the use of a drug. These recommendations vary widely throughout the seven major markets, even for the same drug.
In the US healthcare system, both Public and Private health insurance coverage are included. Also, Medicare and Medicaid are the largest government-funded programs in the US. The major healthcare programs including Medicare, Medicaid, the Children's Health Insurance Program (CHIP), and the state and federal health insurance marketplaces are overseen by the Centers for Medicare & Medicaid Services (CMS). Other than these, Pharmacy Benefit Managers (PBMs), and third-party organizations that provide services, and educational programs to aid patients are also present.
The report further provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of approved therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Scope of the Molecule Glues Market Report
- The Molecule Glues market report covers a segment of key events, an executive summary, and a descriptive overview of the molecule glues, explaining its mechanism, and emerging molecule glues.
- Comprehensive insight into the competitive landscape, and forecasts, the future growth potential of treatment rate, drug uptake, and drug information have been provided.
- Additionally, an all-inclusive account of the current and emerging therapies and the elaborative profiles of late-stage and prominent therapies will impact the current landscape.
- A detailed review of the molecule glues market, historical and forecasted market size, market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The Molecule Glues market report provides an edge while developing business strategies, by understanding trends, through SWOT analysis, expert insights/KOL views, and treatment preferences that help shape and drive the 7MM molecule glues market.
Molecule Glues Market Report Insights
- Molecule Glues Targeted Patient Pool
- Therapeutic Approaches
- Molecule Glues Pipeline Analysis
- Molecule Glues Market Size and Trends
- Existing and future Market Opportunity
Molecule Glues Market Report Key Strengths
- Eleven years Forecast
- The 7MM Coverage
- Key Cross Competition
- Molecule Glues Drugs Uptake
- Key Molecule Glues Market Forecast Assumptions
Molecule Glues Market Report Assessment
- Current Treatment Practices
- Unmet Needs
- Pipeline Product Profiles
- Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
Key Questions answered in the Molecule Glues Market Report
- What was the molecule glues total market size, the market size by therapies, market share (%) distribution, and what would it look like in 2034? What are the contributing factors for this growth?
- Which drug is going to be the largest contributor in 2034?
- Which is the most lucrative market for molecule glues?
- What are the pricing variations among different geographies for approved therapies?
- What are the risks, burdens, and unmet needs of treatment with molecule glues? What will be the growth opportunities across the 7MM for the patient population of molecule glues?
- What are the key factors hampering the growth of the molecule glues market?
- What are the indications for which molecule glues are being developed to overcome the limitations of existing treatments?
- What key designations have been granted to molecule glues?
- Patient acceptability in terms of preferred therapy options as per real-world scenarios?
Reasons to buy Molecule Glues Market Report
- The Molecule Glues market report will help develop business strategies by understanding the latest trends and changing dynamics driving the molecule glues Market.
- Understand the existing market opportunities in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain) the United Kingdom, and Japan.
- Identifying strong upcoming Molecule Glues companies in the market will help devise strategies to help get ahead of competitors.
- Detailed analysis and ranking of indication-wise current and emerging therapies under the conjoint analysis section to provide visibility around leading indications.
- Highlights of Access and Reimbursement policies of approved therapies, barriers to accessibility of expensive off-label therapies, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing market so that the upcoming Molecule Glues companies can strengthen their development and launch strategy.