Menin Inhibitors: A Revolutionary Treatment Paradigm for Acute Leukemia
Feb 06, 2026
Table of Contents
Summary
- Menin inhibitors are an emerging class of targeted therapies that transform treatment for genetically defined acute leukemias, particularly KMT2A‑rearranged and NPM1‑mutated AML.
- REVUFORJ (revumenib, Syndax) is the first FDA‑approved selective menin inhibitor for relapsed/refractory acute leukemia with KMT2A translocation.
- KOMZIFTI (ziftomenib, Kura/Kyowa Kirin) received FDA approval in November 2025 for adults with relapsed/refractory NPM1‑mutated AML with no satisfactory alternatives.
- Additional menin inhibitors, such as Johnson & Johnson’s bleximenib, Sumitomo’s enzomenib (DSP‑5336), Servier’s S243249, and further studies of ziftomenib, are advancing through clinical trials.
Menin inhibitors are becoming an important new class of targeted therapies in oncology, especially for AML. In addition to their role in KMT2A-rearranged leukemia, these agents show potential benefits for patients with NPM1-mutated AML, historically viewed as a favorable-risk group, as well as for leukemias driven by NUP98 or UBTF rearrangements and other fusion abnormalities. They are anticipated to be incorporated into frontline combination treatments, applied as post-transplant maintenance, and possibly included in multi-agent regimens for patients with additional co-mutations.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- Daiichi Sankyo’s Trastuzumab Deruxtecan; ODD to Bexmarilimab for AML; Roche’ Alecensa; Ergomed Ai...
- Tecelra by Adaptimmune: First FDA-Approved Engineered Cell Therapy for Solid Tumors; GSK’s ...
- Actinium Announces SIERRA Trial Results; Santhera Seeks FDA Review for Vamorolone; Seres Announce...
- Sarepta Therapeutics Pauses ELEVIDYS Shipments in U.S. After FDA Intervention Over Patient Deaths...
- FDA Grants Priority Review for Zolbetuximab BLA; FDA Traditional Approval for LEQEMBI for Alzheim...
Rearrangements of the KMT2A gene, located at chromosome locus 11q23, occur in up to 10% of acute leukemias in children and adults, with higher incidence in certain types of infant and childhood acute leukemias. KMT2A rearrangements account for ~5–10% of AML cases and the five-year OS rate for KMT2A rearrangement AML is <20%. AML accounts for about 1 out of 3 leukemias in adults, and for about 1% of all cancers. In 2024, approximately 20,800 new cases of AML were reported in the United States.
The clinical development of menin inhibitors has progressed with remarkable speed over the past several years, driven by compelling preclinical and early clinical data demonstrating encouraging response rates and manageable safety profiles, even in heavily pretreated patients. The approval of the first menin inhibitor marks a significant milestone in precision oncology, particularly for rare genetic subtypes that have historically carried dismal prognoses and limited therapeutic options.
REVUFORJ (Revumenib): The First FDA-Approved Menin Inhibitor
In November 2024, Syndax Pharmaceuticals achieved a landmark milestone with FDA approval of REVUFORJ (revumenib), becoming the first and only selective menin inhibitor approved for clinical use. This approval filled a critical therapeutic gap for patients with relapsed or refractory (R/R) acute leukemia with KMT2A translocation. The approval of REVUFORJ was based on data from the pivotal Phase II trial (NCT04065399), which evaluated revumenib in patients with R/R AML, ALL, and mixed phenotype acute leukemia (MPAL) with KMT2A rearrangements.
REVUFORJ demonstrated a manageable safety profile in the pivotal trial, with differentiation syndrome being the most common Grade ≥3 adverse event, a marker of therapeutic activity rather than off-target toxicity. Most adverse events were manageable with appropriate supportive care and dose modifications. Differentiation syndrome was generally reversible with corticosteroids, and the syndrome’s occurrence often correlated with superior response outcomes.
In June 2025, the FDA granted Priority Review to a supplemental New Drug Application (sNDA) for REVUFORJ for the treatment of R/R NPM1-mutant AML, with a PDUFA target action date of October 25, 2025. This label expansion is expected to significantly broaden the eligible patient population, from 5–10% of AML cases to include an additional 25–30% of patients, thereby dramatically increasing revenue opportunity and patient access.
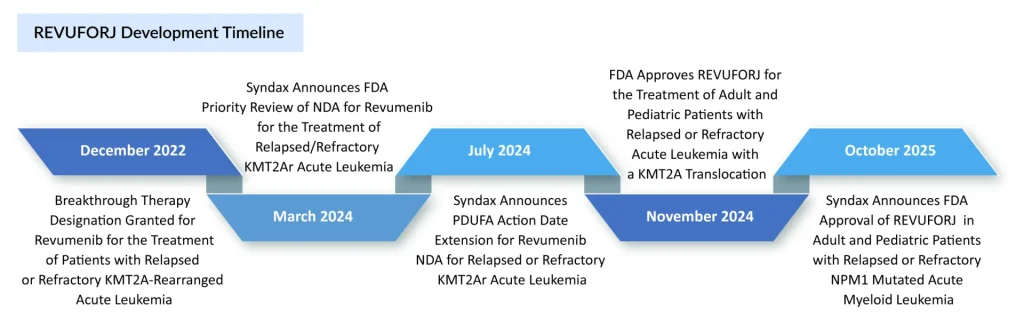
This NPM1 indication approval would position REVUFORJ as the first menin inhibitor with dual-indication approval, providing competitive advantage during the critical phase when emerging competitors enter the market.
REVUFORJ generated USD 7.7 million in net revenue during the fourth quarter of 2024, covering the first five weeks of its U.S. launch. SYNDAX estimates that approximately one-third of this amount is held in inventory by specialty pharmacies and distributors, with the remainder attributable to actual patient use.
In the first half of 2025, REVUFORJ recorded USD 48.6 million in net revenue (USD 20 million in Q1 and USD 28.6 million in Q2). These results demonstrate strong uptake among physicians treating patients with KMT2A-AML, despite treatment interruptions related to transplantation. With the recent label expansion to include NPM1-mutated AML, both the eligible patient population and the revenue potential are expected to increase.
KOMZIFTI: The Newly Approved Menin Inhibitor
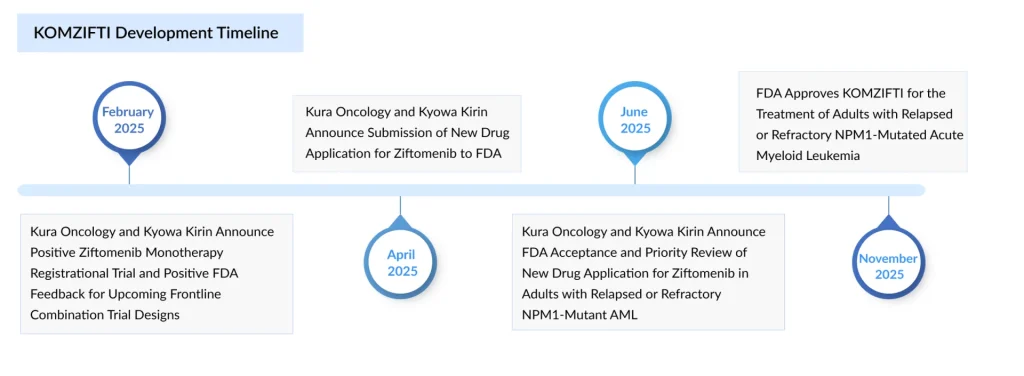
Kyowa Kirin’s major investment in Kura Oncology has proven highly successful, culminating in FDA approval of a new therapy targeting a specific subset of patients with acute myeloid leukemia. In November 2025, the FDA approved Kura’s menin inhibitor, ziftomenib, for adults with R/R AML who carry a susceptible NPM1 mutation and have no suitable alternative treatment options. The drug, which will be sold as KOMZIFTI, received approval based on results from the pivotal KOMET-001 study, in which 21.4% of patients achieved complete remission (CR) or CR with partial hematologic recovery (CRh).
Historically, AML treatment choices for the roughly one-third of patients with NPM1 mutations have been scarce. But in late October, Syndax Pharmaceuticals shifted the landscape when the FDA approved Revuforj as the first menin inhibitor for this population. With both drugs belonging to the same class, KOMZIFTI and REVUFORJ are now positioned to compete head-to-head in this indication.
Emerging Menin Inhibitors: The Competitive Pipeline
While REVUFORJ currently enjoys a first-to-market advantage, newly approved KOMZIFTI is not far behind. In addition to these two, several promising menin inhibitors are advancing through clinical development, each with distinct characteristics, combination strategies, and development timelines.
Johnson & Johnson Innovative Medicine’s Bleximenib
Bleximenib is an experimental oral menin inhibitor currently under investigation for use in both newly diagnosed and relapsed or refractory AML. At the June 2025 European Hematology Association (EHA) Congress, the company presented Phase 1B data on bleximenib combined with venetoclax and azacitidine in an oral session. The findings indicated that the triple combination demonstrated a manageable safety profile, with no evidence of QTc prolongation so far. A 100 mg twice-daily dose of bexarotenib, when administered with venetoclax and azacitidine, produced strong pharmacodynamic activity and greater response depth, consistent with the recommended Phase II monotherapy dose.
Sumitomo Pharma’s Enzomenib
DSP-5336 is an investigational small-molecule agent designed to block the interaction between menin and MLL proteins. Enzomenib received Orphan Drug Designation (ODD) from the FDA for acute myeloid leukemia (AML) in June 2022, followed by Fast Track Designation (FTD) in June 2024 for R/R AML with MLL rearrangements or NPM1 mutations. In September 2024, Japan’s PMDA also granted ODD for enzomenib for R/R AML with MLL rearrangements or mutant NPM1.
In December 2024, early clinical and translational findings from the Phase I/II study of enzomenib were shared at the 66th American Society of Hematology (ASH) Annual Meeting. The safety analysis included 84 patients with acute leukemia, the majority of whom had AML (94%, 79/84). The promising antileukemic activity together with a favorable safety profile indicates that enzomenib could become an important therapeutic option for patients with R/R acute leukemia harboring KMT2A rearrangements or NPM1 mutations.
Servier’s S243249
S243249 (previously BN104) is an innovative, highly potent, and selective small-molecule therapy created by BioNova Pharmaceuticals. Servier obtained the rights to BN104 from BioNova in May 2025. The drug is poised to become a leading menin inhibitor candidate for treating acute leukemias characterized by KMT2A gene rearrangements or NPM1 mutations. Data shared at the 2024 American Society of Hematology (ASH) Annual Meeting showed that patients with relapsed or refractory Acute Myeloid Leukemia (AML) achieved CR/CRh rates of 60.9% in the KMT2A-rearranged group and 40% in the NPM1-mutated group, with a manageable safety profile1 (no QTc prolongation and no grade ≥3 differentiation syndrome). KMT2A rearrangements occur in roughly 5–10%2 of AML cases, while NPM1 mutations appear in about 20–30%3. S243249 received Orphan Drug Designation in April 2023 and Fast Track Designation in October 2023 from the U.S. Food and Drug Administration for the treatment of acute leukemia.
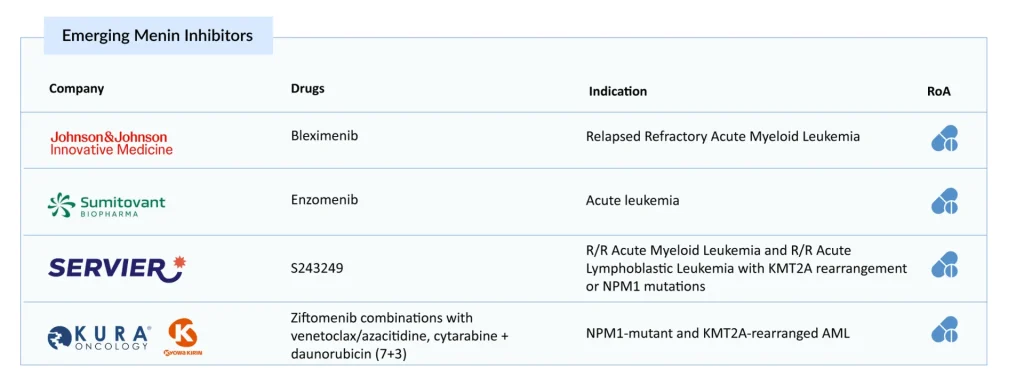
Kura Oncology & Kyowa Kirin’s Ziftomenib
Ziftomenib is an experimental, orally administered menin-KMT2A inhibitor being developed for AML and may be used alongside other targeted treatments. Ziftomenib is currently being evaluated as a monotherapy in the KOMET-001 trial (KMT2A-rearranged ALL and Non-NPM1-mutant/Non-KMT2A-rearranged AML) and in combination with venetoclax/azacitidine, and cytarabine + daunorubicin (7+3) in the KOMET-007 (NPM1-mutant AML and KMT2A-rearranged AML) and combinations with gilteritinib, FLAG-IDA, LDAC in the KOMET-008 (NPM1-mutant AML and KMT2A-rearranged AML) studies. Enrollment for the relapsed/refractory NPM1-mutant AML cohort in the Phase II KOMET-001 trial is complete, and this portion of the study is no longer recruiting.
Conclusion: A Paradigm Shift in Acute Leukemia Treatment
Menin inhibitors represent a watershed moment in the treatment of genetically defined acute leukemias. With REVUFORJ and KOMZIFTI’s FDA approval, this novel class has transitioned from research frontier to clinical reality, offering unprecedented hope for patients with KMT2A-rearranged and NPM1-mutated leukemias who previously faced dismal prognoses.
The menin inhibitor market is poised for rapid growth, driven by the increasing incidence of acute leukemias, particularly AML and ALL, associated with genetic mutations such as KMT2A rearrangements and NPM1 mutations. Favorable regulatory pathways, including FDA Fast Track Designation and Orphan Drug Designation, further accelerate clinical progress by promoting expedited review and earlier market entry. These advantages shorten the timeline from development to patient access, providing a meaningful competitive edge in addressing high-unmet-need conditions such as AML, ALL, and GIST.
In addition, the strong synergistic potential of menin inhibitors when used alongside venetoclax, FLT3 inhibitors, or standard chemotherapy opens opportunities to expand their role across multiple treatment lines. This not only has the potential to improve clinical outcomes but also significantly broadens the overall market opportunity.
The future of menin inhibitor development includes expansion into frontline therapy, maintenance treatment post-transplant, and potential applications in additional malignancies beyond acute leukemias. While challenges remain, including biomarker identification, resistance mechanisms, and optimal patient selection, the rapid progress in this field demonstrates the power of targeted therapy based on sound molecular understanding of disease biology.
For patients with KMT2A-rearranged or NPM1-mutated acute leukemias, menin inhibitors have fundamentally changed the treatment landscape, offering new pathways to remission and improved outcomes. As research continues and clinical experience accumulates, menin inhibitors are poised to become a cornerstone of precision medicine in hematologic malignancies.

Downloads
Article in PDF
Recent Articles
- Actinium Announces Phase III SIERRA Trial Results; FDA Approves Apellis’s Geographic Atrophy Drug...
- FDA Grants Priority Review for Zolbetuximab BLA; FDA Traditional Approval for LEQEMBI for Alzheim...
- Precigen’s PRGN-3006; Yescarta Approved as a First CAR T-cell Therapy for R/R LBCL; Biogen ...
- Novo Nordisk to Acquire Ocedurenone; FDA Awards Orphan Drug Designation to SLS009 in AML; FDA App...
- Nkarta’s Anti-CD19 Allogeneic CAR-NK Cell Therapy, NKX019; Eisai Presents Results of lecanemab fo...




